Treatment of HCV patients before and after renal transplantation
- PMID: 22308151
- PMCID: PMC3269055
- DOI: 10.5812/kowsar.1735143X.712
Treatment of HCV patients before and after renal transplantation
Abstract
Context: Patients with end-stage renal disease can easily acquire a hepatitis C virus (HCV) infection via several ways. An HCV infection is difficult to treat after renal transplantation due to the conflicting actions of immunosuppressant therapy to maintain the function of the transplanted kidney and viricidal interferon (IFN) or ribavirin (RBV) treatment. Antiviral therapy requires great caution to avoid the complex and potentially fatal pharmacological effects. In this review, we examined clinical challenges and potential solutions for this specific scenario.
Evidence acquisitions: We searched Pubmed (NLM), LISTA (EBSCO), Web of Science (TS). The management of patients on waiting list, the indications and regimens about treatment were studied.
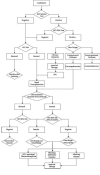
Results: More than forty papers about this topic were found, including seven small clinical trials. International consensus has been reached to test patients awaiting renal transplantation. HCV detection after renal transplantation warrants careful consideration of when to initiate antiviral therapy. Treatment will begin immediately if deteriorating liver function increases the risk for loss of renal function. The choice of regimen depends on the patient's renal function and is individualized under close observation. The immunosuppressive regimen will be adjusted accordingly before antiviral therapy is initiated.
Conclusions: The effects of modified antiviral therapy on these patients varies because of individual characteristics and disease state, and also because of the difficulty associated with conducting a large clinical trial to obtain statistically sound conclusions. The management before transplantation is important and when antiviral therapy needs to start, careful consideration of risks and benefits is needed before initiating this type of treatment.
Keywords: Antiviral Agents; Hepatitis C; Immunosuppression; Kidney Transplantation.
Figures
References
-
- Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2010;80(1):17–28. - PubMed
-
- Ettenger RB, Rosenthal JT, Marik J, Grimm PC, Nelson P, Malekzadeh MH, Fine RN. Long-term results with cyclosporine immune suppression in pediatric cadaver renal transplantation. Transplant Proc. 1991;23(1pt 2):1011–2. - PubMed
-
- Sperl J, Frankova S, Spicak J. [Viral hepatitis in immunosuppressed patients]. Klin Mikrobiol Infekc Lek. 2010;16(4):120–3. - PubMed
-
- Terrault NA, Adey DB. The kidney transplant recipient with hepatitis C infection: pre- and posttransplantation treatment. Clin J Am Soc Nephrol. 2007;2(3):563–75. - PubMed
LinkOut - more resources
Full Text Sources