Reprogrammed keratinocytes from elderly type 2 diabetes patients suppress senescence genes to acquire induced pluripotency
- PMID: 22308265
- PMCID: PMC3292906
- DOI: 10.18632/aging.100428
Reprogrammed keratinocytes from elderly type 2 diabetes patients suppress senescence genes to acquire induced pluripotency
Abstract
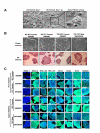
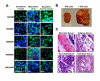
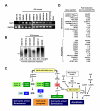
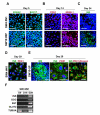
Nuclear reprogramming enables patient-specific derivation of induced pluripotent stem (iPS) cells from adult tissue. Yet, iPS generation from patients with type 2 diabetes (T2D) has not been demonstrated. Here, we report reproducible iPS derivation of epidermal keratinocytes (HK) from elderly T2D patients. Transduced with human OCT4, SOX2, KLF4 and c-MYC stemness factors under serum-free and feeder-free conditions, reprogrammed cells underwent dedifferentiation with mitochondrial restructuring, induction of endogenous pluripotency genes - including NANOG, LIN28, and TERT, and down-regulation of cytoskeletal, MHC class I- and apoptosis-related genes. Notably, derived iPS clones acquired a rejuvenated state, characterized by elongated telomeres and suppressed senescence-related p15INK4b/p16INK4a gene expression and oxidative stress signaling. Stepwise guidance with lineage-specifying factors, including Indolactam V and GLP-1, redifferentiated HK-derived iPS clones into insulin-producing islet-like progeny. Thus, in elderly T2D patients, reprogramming of keratinocytes ensures a senescence-privileged status yielding iPS cells proficient for regenerative applications.
Conflict of interest statement
The authors of this manuscript have no conflict of interest to declare.
Figures


References
-
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. - PubMed
-
- D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. - PubMed
-
- Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, Majumdar AS. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940–1953. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

