CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results
- PMID: 22308288
- PMCID: PMC3350361
- DOI: 10.1182/blood-2011-10-387969
CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results
Abstract
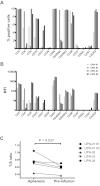
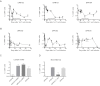
Cellular immune responses have the potential to elicit dramatic and sustained clinical remissions in lymphoma patients. Recent clinical trial data demonstrate that modification of T cells with chimeric antigen receptors (CARs) is a promising strategy. T cells containing CARs with costimulatory domains exhibit improved activity against tumors. We conducted a pilot clinical trial testing a "third-generation" CD20-specific CAR with CD28 and 4-1BB costimulatory domains in patients with relapsed indolent B-cell and mantle cell lymphomas. Four patients were enrolled, and 3 received T-cell infusions after cyclophosphamide lymphodepletion. Treatment was well tolerated, although one patient developed transient infusional symptoms. Two patients without evaluable disease remained progression-free for 12 and 24 months. The third patient had an objective partial remission and relapsed at 12 months after infusions. Modified T cells were detected by quantitative PCR at tumor sites and up to 1 year in peripheral blood, albeit at low levels. No evidence of host immune responses against infused cells was detected. In conclusion, adoptive immunotherapy with CD20-specific T cells was well tolerated and was associated with antitumor activity. We will pursue alternative gene transfer technologies and culture conditions in future studies to improve CAR expression and cell production efficiency.
Trial registration: ClinicalTrials.gov NCT00621452.
Figures



Comment in
-
CARs and cancers: questions and answers.Blood. 2012 Apr 26;119(17):3872-3. doi: 10.1182/blood-2012-02-410373. Blood. 2012. PMID: 22538493
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

