Active transcription and essential role of RNA polymerase II at the centromere during mitosis
- PMID: 22308327
- PMCID: PMC3277563
- DOI: 10.1073/pnas.1108705109
Active transcription and essential role of RNA polymerase II at the centromere during mitosis
Abstract
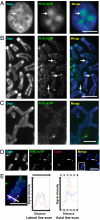
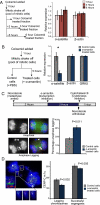
Transcription of the centromeric regions has been reported to occur in G1 and S phase in different species. Here, we investigate whether transcription also occurs and plays a functional role at the mammalian centromere during mitosis. We show the presence of actively transcribing RNA polymerase II (RNAPII) and its associated transcription factors, coupled with the production of centromere satellite transcripts at the mitotic kinetochore. Specific inhibition of RNAPII activity during mitosis leads to a decrease in centromeric α-satellite transcription and a concomitant increase in anaphase-lagging cells, with the lagging chromosomes showing reduced centromere protein C binding. These findings demonstrate an essential role of RNAPII in the transcription of α-satellite DNA, binding of centromere protein C, and the proper functioning of the mitotic kinetochore.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

