Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase
- PMID: 22308364
- PMCID: PMC3289319
- DOI: 10.1073/pnas.1113873109
Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase
Abstract
Tryptophan catabolism mediated by indoleamine 2,3-dioxygenase (IDO1) is an important mechanism of peripheral immune tolerance contributing to tumoral immune resistance, and IDO1 inhibition is an active area of drug development. Tryptophan 2,3-dioxygenase (TDO) is an unrelated hepatic enzyme that also degrades tryptophan along the kynurenine pathway. Here, we show that enzymatically active TDO is expressed in a significant proportion of human tumors. In a preclinical model, TDO expression by tumors prevented their rejection by immunized mice. We developed a TDO inhibitor, which, upon systemic treatment, restored the ability of mice to reject TDO-expressing tumors. Our results describe a mechanism of tumoral immune resistance based on TDO expression and establish proof-of-concept for the use of TDO inhibitors in cancer therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
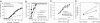
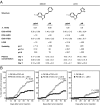
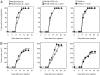
References
-
- Munn DH, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. - PubMed
-
- Uyttenhove C, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. - PubMed
-
- Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

