Actin filament curvature biases branching direction
- PMID: 22308368
- PMCID: PMC3286980
- DOI: 10.1073/pnas.1114292109
Actin filament curvature biases branching direction
Abstract
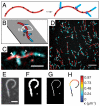
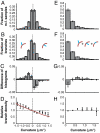
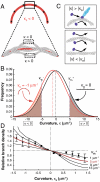
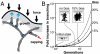
Mechanical cues affect many important biological processes in metazoan cells, such as migration, proliferation, and differentiation. Such cues are thought to be detected by specialized mechanosensing molecules linked to the cytoskeleton, an intracellular network of protein filaments that provide mechanical rigidity to the cell and drive cellular shape change. The most abundant such filament, actin, forms branched networks nucleated by the actin-related protein (Arp) 2/3 complex that support or induce membrane protrusions and display adaptive behavior in response to compressive forces. Here we show that filamentous actin serves in a mechanosensitive capacity itself, by biasing the location of actin branch nucleation in response to filament bending. Using an in vitro assay to measure branching from curved sections of immobilized actin filaments, we observed preferential branch formation by the Arp2/3 complex on the convex face of the curved filament. To explain this behavior, we propose a fluctuation gating model in which filament binding or branch nucleation by Arp2/3 occur only when a sufficiently large, transient, local curvature fluctuation causes a favorable conformational change in the filament, and we show with Monte Carlo simulations that this model can quantitatively account for our experimental data. We also show how the branching bias can reinforce actin networks in response to compressive forces. These results demonstrate how filament curvature can alter the interaction of cytoskeletal filaments with regulatory proteins, suggesting that direct mechanotransduction by actin may serve as a general mechanism for organizing the cytoskeleton in response to force.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Actin bends over backward for directional branching.Proc Natl Acad Sci U S A. 2012 Feb 21;109(8):2693-4. doi: 10.1073/pnas.1121360109. Epub 2012 Feb 9. Proc Natl Acad Sci U S A. 2012. PMID: 22323602 Free PMC article. No abstract available.
References
-
- Farge E. Mechanotransduction in development. Curr Top Dev Biol. 2011;95:243–265. - PubMed
-
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. - PubMed
-
- Janmey PA, McCulloch CA. Cell mechanics: Integrating cell responses to mechanical stimuli. Annu Rev Biomed Eng. 2007;9:1–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

