Glucose activates free fatty acid receptor 1 gene transcription via phosphatidylinositol-3-kinase-dependent O-GlcNAcylation of pancreas-duodenum homeobox-1
- PMID: 22308370
- PMCID: PMC3289358
- DOI: 10.1073/pnas.1114350109
Glucose activates free fatty acid receptor 1 gene transcription via phosphatidylinositol-3-kinase-dependent O-GlcNAcylation of pancreas-duodenum homeobox-1
Abstract
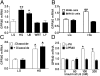
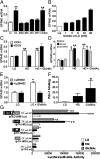
The G protein-coupled free fatty acid receptor-1 (FFA1/GPR40) plays a major role in the regulation of insulin secretion by fatty acids. GPR40 is considered a potential therapeutic target to enhance insulin secretion in type 2 diabetes; however, its mode of regulation is essentially unknown. The aims of this study were to test the hypothesis that glucose regulates GPR40 gene expression in pancreatic β-cells and to determine the mechanisms of this regulation. We observed that glucose stimulates GPR40 gene transcription in pancreatic β-cells via increased binding of pancreas-duodenum homeobox-1 (Pdx-1) to the A-box in the HR2 region of the GPR40 promoter. Mutation of the Pdx-1 binding site within the HR2 abolishes glucose activation of GPR40 promoter activity. The stimulation of GPR40 expression and Pdx-1 binding to the HR2 in response to glucose are mimicked by N-acetyl glucosamine, an intermediate of the hexosamine biosynthesis pathway, and involve PI3K-dependent O-GlcNAcylation of Pdx-1 in the nucleus. We demonstrate that O-GlcNAc transferase (OGT) interacts with the product of the PI3K reaction, phosphatidylinositol 3,4,5-trisphosphate (PIP(3)), in the nucleus. This interaction enables OGT to catalyze O-GlcNAcylation of nuclear proteins, including Pdx-1. We conclude that glucose stimulates GPR40 gene expression at the transcriptional level through Pdx-1 binding to the HR2 region and via a signaling cascade that involves an interaction between OGT and PIP(3) at the nuclear membrane. These observations reveal a unique mechanism by which glucose metabolism regulates the function of transcription factors in the nucleus to induce gene expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Elevation of the post-translational modification of proteins by O-linked N-acetylglucosamine leads to deterioration of the glucose-stimulated insulin secretion in the pancreas of diabetic Goto-Kakizaki rats.Glycobiology. 2007 Feb;17(2):127-40. doi: 10.1093/glycob/cwl067. Epub 2006 Nov 9. Glycobiology. 2007. PMID: 17095531
-
PPAR-γ activation increases insulin secretion through the up-regulation of the free fatty acid receptor GPR40 in pancreatic β-cells.PLoS One. 2013;8(1):e50128. doi: 10.1371/journal.pone.0050128. Epub 2013 Jan 23. PLoS One. 2013. PMID: 23372643 Free PMC article.
-
The transcription factor PDX-1 is post-translationally modified by O-linked N-acetylglucosamine and this modification is correlated with its DNA binding activity and insulin secretion in min6 beta-cells.Arch Biochem Biophys. 2003 Jul 15;415(2):155-63. doi: 10.1016/s0003-9861(03)00234-0. Arch Biochem Biophys. 2003. PMID: 12831837
-
[A New Pain Regulatory System via the Brain Long Chain Fatty Acid Receptor GPR40/FFA1 Signal].Yakugaku Zasshi. 2017;137(2):199-204. doi: 10.1248/yakushi.16-00208. Yakugaku Zasshi. 2017. PMID: 28154332 Review. Japanese.
-
The fatty acid receptor FFA1/GPR40 a decade later: how much do we know?Trends Endocrinol Metab. 2013 Aug;24(8):398-407. doi: 10.1016/j.tem.2013.03.003. Epub 2013 Apr 27. Trends Endocrinol Metab. 2013. PMID: 23631851 Review.
Cited by
-
Omega-3 Fatty Acids as Druggable Therapeutics for Neurodegenerative Disorders.CNS Neurol Disord Drug Targets. 2019;18(10):735-749. doi: 10.2174/1871527318666191114093749. CNS Neurol Disord Drug Targets. 2019. PMID: 31724519 Free PMC article. Review.
-
Role of nutrient-driven O-GlcNAc-post-translational modification in pancreatic exocrine and endocrine islet development.Development. 2020 Apr 12;147(7):dev186643. doi: 10.1242/dev.186643. Development. 2020. PMID: 32165492 Free PMC article.
-
Targeting protein modifications in metabolic diseases: molecular mechanisms and targeted therapies.Signal Transduct Target Ther. 2023 May 27;8(1):220. doi: 10.1038/s41392-023-01439-y. Signal Transduct Target Ther. 2023. PMID: 37244925 Free PMC article. Review.
-
Engineered commensal bacteria reprogram intestinal cells into glucose-responsive insulin-secreting cells for the treatment of diabetes.Diabetes. 2015 May;64(5):1794-803. doi: 10.2337/db14-0635. Epub 2015 Jan 27. Diabetes. 2015. PMID: 25626737 Free PMC article.
-
Free fatty acid receptor 1: a ray of hope in the therapy of type 2 diabetes mellitus.Inflammopharmacology. 2021 Dec;29(6):1625-1639. doi: 10.1007/s10787-021-00879-8. Epub 2021 Oct 20. Inflammopharmacology. 2021. PMID: 34669065 Review.
References
-
- Poitout V. Lipid partitioning in the pancreatic beta-cell: Physiologic and pathophysiologic implications. Curr Opin Endocrinol Diabetes. 2002;9:152–159.
-
- Itoh Y, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. - PubMed
-
- Briscoe CP, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278:11303–11311. - PubMed
-
- Kotarsky K, Nilsson NE, Flodgren E, Owman C, Olde B. A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem Biophys Res Commun. 2003;301:406–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

