Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection
- PMID: 22308386
- PMCID: PMC3289300
- DOI: 10.1073/pnas.1115369109
Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection
Abstract
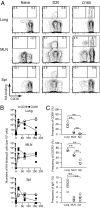
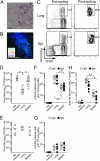
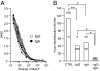
After pulmonary virus infection, virus-binding B cells ectopically accumulate in the lung. However, their contribution to protective immunity against reinfecting viruses remains unknown. Here, we show the phenotypes and protective functions of virus-binding memory B cells that persist in the lung following pulmonary infection with influenza virus. A fraction of virus-binding B-cell population in the lung expressed surface markers for splenic mature memory B cells (CD73, CD80, and CD273) along with CD69 and CXCR3 that are up-regulated on lung effector/memory T cells. The lung B-cell population with memory phenotype persisted for more than 5 mo after infection, and on reinfection promptly differentiated into plasma cells that produced virus-neutralizing antibodies locally. This production of local IgG and IgA neutralizing antibody was correlated with reduced virus spread in adapted hosts. Our data demonstrates that infected lungs harbor a memory B-cell subset with distinctive phenotype and ability to provide protection against pulmonary virus reinfection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Dörner T, Radbruch A. Antibodies and B cell memory in viral immunity. Immunity. 2007;27:384–392. - PubMed
-
- Coro ES, Chang WL, Baumgarth N. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J Immunol. 2006;176:4343–4351. - PubMed
-
- Moyron-Quiroz JE, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–934. - PubMed
-
- Jones PD, Ada GL. Persistence of influenza virus-specific antibody-secreting cells and B cell memory after primary murine influenza virus infection. Cell Immunol. 1987;109:53–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

