Gating transitions in the selectivity filter region of a sodium channel are coupled to the domain IV voltage sensor
- PMID: 22308389
- PMCID: PMC3289344
- DOI: 10.1073/pnas.1210413109
Gating transitions in the selectivity filter region of a sodium channel are coupled to the domain IV voltage sensor
Abstract
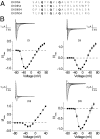
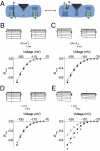
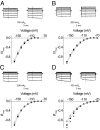
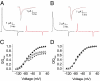
Voltage-dependent ion channels are crucial for generation and propagation of electrical activity in biological systems. The primary mechanism for voltage transduction in these proteins involves the movement of a voltage-sensing domain (D), which opens a gate located on the cytoplasmic side. A distinct conformational change in the selectivity filter near the extracellular side has been implicated in slow inactivation gating, which is important for spike frequency adaptation in neural circuits. However, it remains an open question whether gating transitions in the selectivity filter region are also actuated by voltage sensors. Here, we examine conformational coupling between each of the four voltage sensors and the outer pore of a eukaryotic voltage-dependent sodium channel. The voltage sensors of these sodium channels are not structurally symmetric and exhibit functional specialization. To track the conformational rearrangements of individual voltage-sensing domains, we recorded domain-specific gating pore currents. Our data show that, of the four voltage sensors, only the domain IV voltage sensor is coupled to the conformation of the selectivity filter region of the sodium channel. Trapping the outer pore in a particular conformation with a high-affinity toxin or disulphide crossbridge impedes the return of this voltage sensor to its resting conformation. Our findings directly establish that, in addition to the canonical electromechanical coupling between voltage sensor and inner pore gates of a sodium channel, gating transitions in the selectivity filter region are also coupled to the movement of a voltage sensor. Furthermore, our results also imply that the voltage sensor of domain IV is unique in this linkage and in the ability to initiate slow inactivation in sodium channels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Liu Y, Holmgren M, Jurman ME, Yellen G. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 1997;19:175–184. - PubMed
-
- Jiang Y, et al. The open pore conformation of potassium channels. Nature. 2002;417:523–526. - PubMed
-
- Liu Y, Jurman ME, Yellen G. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron. 1996;16:859–867. - PubMed
-
- López-Barneo J, Hoshi T, Heinemann SH, Aldrich RW. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1993;1:61–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

