Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection
- PMID: 22308390
- PMCID: PMC3277520
- DOI: 10.1073/pnas.1115621109
Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection
Abstract
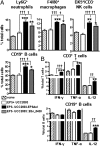
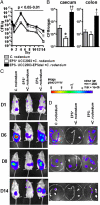
Bifidobacteria comprise a significant proportion of the human gut microbiota. Several bifidobacterial strains are currently used as therapeutic interventions, claiming various health benefits by acting as probiotics. However, the precise mechanisms by which they maintain habitation within their host and consequently provide these benefits are not fully understood. Here we show that Bifidobacterium breve UCC2003 produces a cell surface-associated exopolysaccharide (EPS), the biosynthesis of which is directed by either half of a bidirectional gene cluster, thus leading to production of one of two possible EPSs. Alternate transcription of the two opposing halves of this cluster appears to be the result of promoter reorientation. Surface EPS provided stress tolerance and promoted in vivo persistence, but not initial colonization. Marked differences were observed in host immune response: strains producing surface EPS (EPS(+)) failed to elicit a strong immune response compared with EPS-deficient variants. Specifically, EPS production was shown to be linked to the evasion of adaptive B-cell responses. Furthermore, presence of EPS(+) B. breve reduced colonization levels of the gut pathogen Citrobacter rodentium. Our data thus assigns a pivotal and beneficial role for EPS in modulating various aspects of bifidobacterial-host interaction, including the ability of commensal bacteria to remain immunologically silent and in turn provide pathogen protection. This finding enforces the probiotic concept and provides mechanistic insights into health-promoting benefits for both animal and human hosts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
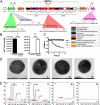
 represents a number of genes between Bbr_0451 and Bbr_0462 or between Bbr_463 and Bbr_0474. Transcriptional start sites, −10 and −35 sites of promoters are indicated; the color coding of the genes relates to their predicted function as indicated in the inset. (B) qRT-PCR analysis of the Bbr_0441 and Bbr_0442 transcriptional units. (I) Transcription of Bbr_0441 in strains UCC2003 and UCC2003-EPS Inv; (II) transcription of Bbr_0442 in UCC2003 and UCC2003-EPS Inv. (C) OD measurements (OD600nm) of UCC2003 and its derivatives over a 5.5-h time period grown in batch culture without agitation; the observed drop in OD values for the EPS− derivatives is because of cell sedimentation. (D) Transmission electron microscopy images of B. breve UCC2003 (I) and isogenic derivatives UCC2003-EPSInv (II), UCC2003-EPSdel (III), UCC2003::Bbr_0430 (IV). (Scale bars, 100 nm.) (E) High performance anion-exchange chromatography with pulsed amperometric detection profiles of acid-hydrolyzed surface EPS isolated from UCC2003 and isogenic derivatives (see D for strain coding).
represents a number of genes between Bbr_0451 and Bbr_0462 or between Bbr_463 and Bbr_0474. Transcriptional start sites, −10 and −35 sites of promoters are indicated; the color coding of the genes relates to their predicted function as indicated in the inset. (B) qRT-PCR analysis of the Bbr_0441 and Bbr_0442 transcriptional units. (I) Transcription of Bbr_0441 in strains UCC2003 and UCC2003-EPS Inv; (II) transcription of Bbr_0442 in UCC2003 and UCC2003-EPS Inv. (C) OD measurements (OD600nm) of UCC2003 and its derivatives over a 5.5-h time period grown in batch culture without agitation; the observed drop in OD values for the EPS− derivatives is because of cell sedimentation. (D) Transmission electron microscopy images of B. breve UCC2003 (I) and isogenic derivatives UCC2003-EPSInv (II), UCC2003-EPSdel (III), UCC2003::Bbr_0430 (IV). (Scale bars, 100 nm.) (E) High performance anion-exchange chromatography with pulsed amperometric detection profiles of acid-hydrolyzed surface EPS isolated from UCC2003 and isogenic derivatives (see D for strain coding).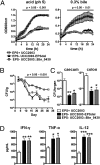

References
-
- Kelly D, Conway S, Aminov R. Commensal gut bacteria: Mechanisms of immune modulation. Trends Immunol. 2005;26:326–333. - PubMed
-
- WHO 2001. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria (World Health Organization, Geneva). www.who.int/entity/foodsafety/publications/fs_management/en/probiotics.pdf. Accessed January 10, 2012.
-
- Coakley M, et al. Inhibitory effect of conjugated alpha-linolenic acid from Bifidobacteria of intestinal origin on SW480 cancer cells. Lipids. 2009;44:249–256. - PubMed
-
- Fukuda S, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

