Identification of residues defining phospholipid flippase substrate specificity of type IV P-type ATPases
- PMID: 22308393
- PMCID: PMC3277569
- DOI: 10.1073/pnas.1115725109
Identification of residues defining phospholipid flippase substrate specificity of type IV P-type ATPases
Abstract
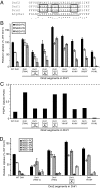
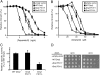
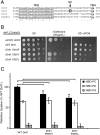
Type IV P-type ATPases (P4-ATPases) catalyze translocation of phospholipid across a membrane to establish an asymmetric bilayer structure with phosphatidylserine (PS) and phosphatidylethanolamine (PE) restricted to the cytosolic leaflet. The mechanism for how P4-ATPases recognize and flip phospholipid is unknown, and is described as the "giant substrate problem" because the canonical substrate binding pockets of homologous cation pumps are too small to accommodate a bulky phospholipid. Here, we identify residues that confer differences in substrate specificity between Drs2 and Dnf1, Saccharomyces cerevisiae P4-ATPases that preferentially flip PS and phosphatidylcholine (PC), respectively. Transplanting transmembrane segments 3 and 4 (TM3-4) of Drs2 into Dnf1 alters the substrate preference of Dnf1 from PC to PS. Acquisition of the PS substrate maps to a Tyr618Phe substitution in TM4 of Dnf1, representing the loss of a single hydroxyl group. The reciprocal Phe511Tyr substitution in Drs2 specifically abrogates PS recognition by this flippase causing PS exposure on the outer leaflet of the plasma membrane without disrupting PE asymmetry. TM3 and the adjoining lumenal loop contribute residues important for Dnf1 PC preference, including Phe587. Modeling of residues involved in substrate selection suggests a novel P-type ATPase transport pathway at the protein/lipid interface and a potential solution to the giant substrate problem.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Conserved mechanism of phospholipid substrate recognition by the P4-ATPase Neo1 from Saccharomyces cerevisiae.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Feb;1865(2):158581. doi: 10.1016/j.bbalip.2019.158581. Epub 2019 Nov 28. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 31786280 Free PMC article.
-
Type IV P-type ATPases distinguish mono- versus diacyl phosphatidylserine using a cytofacial exit gate in the membrane domain.J Biol Chem. 2013 Jul 5;288(27):19516-27. doi: 10.1074/jbc.M113.476911. Epub 2013 May 24. J Biol Chem. 2013. PMID: 23709217 Free PMC article.
-
Two-gate mechanism for phospholipid selection and transport by type IV P-type ATPases.Proc Natl Acad Sci U S A. 2013 Jan 29;110(5):E358-67. doi: 10.1073/pnas.1216948110. Epub 2013 Jan 9. Proc Natl Acad Sci U S A. 2013. PMID: 23302692 Free PMC article.
-
Substrates of P4-ATPases: beyond aminophospholipids (phosphatidylserine and phosphatidylethanolamine).FASEB J. 2019 Mar;33(3):3087-3096. doi: 10.1096/fj.201801873R. Epub 2018 Dec 3. FASEB J. 2019. PMID: 30509129 Review.
-
Linking phospholipid flippases to vesicle-mediated protein transport.Biochim Biophys Acta. 2009 Jul;1791(7):612-9. doi: 10.1016/j.bbalip.2009.03.004. Epub 2009 Mar 12. Biochim Biophys Acta. 2009. PMID: 19286470 Free PMC article. Review.
Cited by
-
Lipid Transport by Candida albicans Dnf2 Is Required for Hyphal Growth and Virulence.Infect Immun. 2022 Nov 17;90(11):e0041622. doi: 10.1128/iai.00416-22. Epub 2022 Oct 10. Infect Immun. 2022. PMID: 36214556 Free PMC article.
-
Loss of the Arabidopsis thaliana P4-ATPases ALA6 and ALA7 impairs pollen fitness and alters the pollen tube plasma membrane.Front Plant Sci. 2015 Apr 21;6:197. doi: 10.3389/fpls.2015.00197. eCollection 2015. Front Plant Sci. 2015. PMID: 25954280 Free PMC article.
-
Decoding P4-ATPase substrate interactions.Crit Rev Biochem Mol Biol. 2016 Nov/Dec;51(6):513-527. doi: 10.1080/10409238.2016.1237934. Epub 2016 Oct 4. Crit Rev Biochem Mol Biol. 2016. PMID: 27696908 Free PMC article. Review.
-
The PQ-loop protein Any1 segregates Drs2 and Neo1 functions required for viability and plasma membrane phospholipid asymmetry.J Lipid Res. 2019 May;60(5):1032-1042. doi: 10.1194/jlr.M093526. Epub 2019 Mar 1. J Lipid Res. 2019. PMID: 30824614 Free PMC article.
-
Conserved mechanism of phospholipid substrate recognition by the P4-ATPase Neo1 from Saccharomyces cerevisiae.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Feb;1865(2):158581. doi: 10.1016/j.bbalip.2019.158581. Epub 2019 Nov 28. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 31786280 Free PMC article.
References
-
- Axelsen KB, Palmgren MG. Evolution of substrate specificities in the P-Type ATPase superfamily. J Mol Evol. 1998;46:84–101. - PubMed
-
- Toyoshima C. Structural aspects of ion pumping by Ca2+-ATPase of sarcoplasmic reticulum. Arch Biochem Biophys. 2008;476:3–11. - PubMed
-
- Shinoda T, Ogawa H, Cornelius F, Toyoshima C. Crystal structure of the sodium-potassium pump at 2.4 A resolution. Nature. 2009;459:446–450. - PubMed
-
- Morth JP, et al. A structural overview of the plasma membrane Na+,K+-ATPase and H+-ATPase ion pumps. Nat Rev Mol Cell Biol. 2011;12:60–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

