Structure of saposin A lipoprotein discs
- PMID: 22308394
- PMCID: PMC3286916
- DOI: 10.1073/pnas.1115743109
Structure of saposin A lipoprotein discs
Abstract
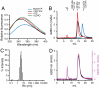
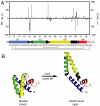
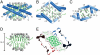
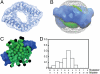
The saposins are small, membrane-active proteins that exist in both soluble and lipid-bound states. Saposin A has roles in sphingolipid catabolism and transport and is required for the breakdown of galactosylceramide by β-galactosylceramidase. In the absence of lipid, saposin A adopts a closed monomeric apo conformation typical of this family. To study a lipid-bound state of this protein, we determined the crystal structure of saposin A in the presence of detergent to 1.9 Å resolution. The structure reveals two chains of saposin A in an open conformation encapsulating 40 internally bound detergent molecules organized in a highly ordered bilayer-like hydrophobic core. The complex provides a high-resolution view of a discoidal lipoprotein particle in which all of the internalized acyl chains are resolved. Saposin A lipoprotein discs exhibit limited selectivity with respect to the incorporated lipid, and can solubilize phospholipids, sphingolipids, and cholesterol into discrete, monodisperse particles with mass of approximately 27 kDa. These discs may be the smallest possible lipoprotein structures that are stabilized by lipid self-assembly.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kolter T, Sandhoff K. Lysosomal degradation of membrane lipids. FEBS Lett. 2010;584:1700–1712. - PubMed
-
- Wendeler M, et al. Photoaffinity labelling of the human GM2-activator protein. Mechanistic insight into ganglioside GM2 degradation. Eur J Biochem. 2004;271:614–627. - PubMed
-
- O’Brien JS, et al. Coding of two sphingolipid activator proteins (SAP-1 and SAP-2) by same genetic locus. Science. 1988;241:1098–1101. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

