Global kinetic analysis of proteolysis via quantitative targeted proteomics
- PMID: 22308409
- PMCID: PMC3277568
- DOI: 10.1073/pnas.1117158109
Global kinetic analysis of proteolysis via quantitative targeted proteomics
Abstract
Mass spectrometry-based proteomics is a powerful tool for identifying hundreds to thousands of posttranslational modifications in complex mixtures. However, it remains enormously challenging to simultaneously assess the intrinsic catalytic efficiencies (k(cat)/K(M)) of these modifications in the context of their natural interactors. Such fundamental enzymological constants are key to determining substrate specificity and for establishing the timing and importance of cellular signaling. Here, we report the use of selected reaction monitoring (SRM) for tracking proteolysis induced by human apoptotic caspases-3, -7, -8, and -9 in lysates and living cells. By following the appearance of the cleaved peptides in lysate as a function of time, we were able to determine hundreds of catalytic efficiencies in parallel. Remarkably, we find the rates of substrate hydrolysis for individual caspases vary greater than 500-fold indicating a sequential process. Moreover, the rank-order of substrate cutting is similar in apoptotic cells, suggesting that cellular structures do not dramatically alter substrate accessibility. Comparisons of extrinsic (TRAIL) and intrinsic (staurosporine) inducers of apoptosis revealed similar substrate profiles, suggesting the final proteolytic demolitions proceed by similarly ordered plans. Certain biological processes were rapidly targeted by the caspases, including multiple components of the endocyotic pathway and miRNA processing machinery. We believe this massively parallel and quantitative label-free approach to obtaining basic enzymological constants will facilitate the study of proteolysis and other posttranslational modifications in complex mixtures.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


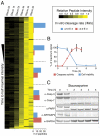
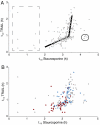
References
-
- Crawford ED, Wells JA. Caspase substrates and cellular remodeling. Annu Rev Biochem. 2011;80:1055–1087. - PubMed
-
- Van Damme P, et al. Caspase-specific and nonspecific in vivo protein processing during Fas-induced apoptosis. Nat Methods. 2005;2:771–777. - PubMed
-
- Lüthi AU, Martin SJ. The CASBAH: A searchable database of caspase substrates. Cell Death Differ. 2007;14:641–650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

