The protein SdhA maintains the integrity of the Legionella-containing vacuole
- PMID: 22308473
- PMCID: PMC3295292
- DOI: 10.1073/pnas.1121286109
The protein SdhA maintains the integrity of the Legionella-containing vacuole
Abstract
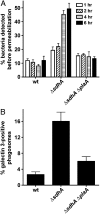
Legionella pneumophila directs the formation of a specialized vacuole within host cells, dependent on protein substrates of the Icm/Dot translocation system. Survival of the host cell is essential for intracellular replication of L. pneumophila. Strains lacking the translocated substrate SdhA are defective for intracellular replication and activate host cell death pathways in primary macrophages. To understand how SdhA promotes evasion of death pathways, we performed a mutant hunt to identify bacterial suppressors of the ΔsdhA growth defect. We identified the secreted phospholipase PlaA as key to activation of death pathways by the ΔsdhA strain. Based on homology between PlaA and SseJ, a Salmonella protein associated with vacuole degradation, we determined the roles of SdhA and PlaA in controlling vacuole integrity. In the absence of sdhA, the Legionella-containing vacuole was unstable, resulting in access to the host cytosol. Both vacuole disruption and host cell death were largely dependent on PlaA. Consistent with these observations, the ΔsdhA strain colocalized with galectin-3, a marker of vacuole rupture, in a PlaA-dependent process. Access of ΔsdhA strains to the macrophage cytosol triggered multiple responses in the host cell, including degradation of bacteria, induction of the type I IFN response, and activation of inflammasomes. Therefore, we have demonstrated that the Legionella-containing vacuole is actively stabilized by the SdhA protein during intracellular replication. This vacuolar niche affords the bacterium protection from cytosolic host factors that degrade bacteria and initiate immune responses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Vacuolar pathogens value membrane integrity.Proc Natl Acad Sci U S A. 2012 Feb 28;109(9):3197-8. doi: 10.1073/pnas.1200326109. Epub 2012 Feb 16. Proc Natl Acad Sci U S A. 2012. PMID: 22343287 Free PMC article. No abstract available.
Similar articles
-
Components of the endocytic and recycling trafficking pathways interfere with the integrity of the Legionella-containing vacuole.Cell Microbiol. 2020 Apr;22(4):e13151. doi: 10.1111/cmi.13151. Cell Microbiol. 2020. PMID: 32096265 Free PMC article.
-
Preventing bacterial DNA release and absent in melanoma 2 inflammasome activation by a Legionella effector functioning in membrane trafficking.Proc Natl Acad Sci U S A. 2012 Apr 17;109(16):6193-8. doi: 10.1073/pnas.1117490109. Epub 2012 Apr 2. Proc Natl Acad Sci U S A. 2012. PMID: 22474394 Free PMC article.
-
SdhA blocks disruption of the Legionella-containing vacuole by hijacking the OCRL phosphatase.Cell Rep. 2021 Nov 2;37(5):109894. doi: 10.1016/j.celrep.2021.109894. Cell Rep. 2021. PMID: 34731604 Free PMC article.
-
The Legionella pneumophila replication vacuole: making a cosy niche inside host cells.Nat Rev Microbiol. 2009 Jan;7(1):13-24. doi: 10.1038/nrmicro1967. Epub 2008 Nov 17. Nat Rev Microbiol. 2009. PMID: 19011659 Free PMC article. Review.
-
Autophagy Evasion and Endoplasmic Reticulum Subversion: The Yin and Yang of Legionella Intracellular Infection.Annu Rev Microbiol. 2016 Sep 8;70:413-33. doi: 10.1146/annurev-micro-102215-095557. Annu Rev Microbiol. 2016. PMID: 27607556 Review.
Cited by
-
Caspase Exploitation by Legionella pneumophila.Front Microbiol. 2016 Apr 13;7:515. doi: 10.3389/fmicb.2016.00515. eCollection 2016. Front Microbiol. 2016. PMID: 27148204 Free PMC article. Review.
-
Identification of a Transcription Factor That Regulates Host Cell Exit and Virulence of Mycobacterium tuberculosis.PLoS Pathog. 2016 May 18;12(5):e1005652. doi: 10.1371/journal.ppat.1005652. eCollection 2016 May. PLoS Pathog. 2016. PMID: 27191591 Free PMC article.
-
Amoebae as training grounds for microbial pathogens.mBio. 2024 Aug 14;15(8):e0082724. doi: 10.1128/mbio.00827-24. Epub 2024 Jul 8. mBio. 2024. PMID: 38975782 Free PMC article. Review.
-
Listeria monocytogenes GlmR Is an Accessory Uridyltransferase Essential for Cytosolic Survival and Virulence.mBio. 2023 Apr 25;14(2):e0007323. doi: 10.1128/mbio.00073-23. Epub 2023 Mar 20. mBio. 2023. PMID: 36939339 Free PMC article.
-
The common HAQ STING variant impairs cGAS-dependent antibacterial responses and is associated with susceptibility to Legionnaires' disease in humans.PLoS Pathog. 2018 Jan 3;14(1):e1006829. doi: 10.1371/journal.ppat.1006829. eCollection 2018 Jan. PLoS Pathog. 2018. PMID: 29298342 Free PMC article. Clinical Trial.
References
-
- Vogel JP, Andrews HL, Wong SK, Isberg RR. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

