Reduced release probability prevents vesicle depletion and transmission failure at dynamin mutant synapses
- PMID: 22308498
- PMCID: PMC3286982
- DOI: 10.1073/pnas.1121626109
Reduced release probability prevents vesicle depletion and transmission failure at dynamin mutant synapses
Abstract
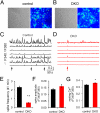
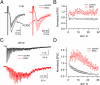
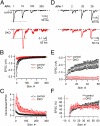
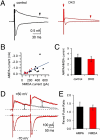
Endocytic recycling of synaptic vesicles after exocytosis is critical for nervous system function. At synapses of cultured neurons that lack the two "neuronal" dynamins, dynamin 1 and 3, smaller excitatory postsynaptic currents are observed due to an impairment of the fission reaction of endocytosis that results in an accumulation of arrested clathrin-coated pits and a greatly reduced synaptic vesicle number. Surprisingly, despite a smaller readily releasable vesicle pool and fewer docked vesicles, a strong facilitation, which correlated with lower vesicle release probability, was observed upon action potential stimulation at such synapses. Furthermore, although network activity in mutant cultures was lower, Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) activity was unexpectedly increased, consistent with the previous report of an enhanced state of synapsin 1 phosphorylation at CaMKII-dependent sites in such neurons. These changes were partially reversed by overnight silencing of synaptic activity with tetrodotoxin, a treatment that allows progression of arrested endocytic pits to synaptic vesicles. Facilitation was also counteracted by CaMKII inhibition. These findings reveal a mechanism aimed at preventing synaptic transmission failure due to vesicle depletion when recycling vesicle traffic is backed up by a defect in dynamin-dependent endocytosis and provide new insight into the coupling between endocytosis and exocytosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Dynamin and activity regulate synaptic vesicle recycling in sympathetic neurons.J Biol Chem. 2009 Jan 16;284(3):1930-7. doi: 10.1074/jbc.M803691200. Epub 2008 Nov 14. J Biol Chem. 2009. PMID: 19010787
-
Inhibition of dynamin completely blocks compensatory synaptic vesicle endocytosis.Proc Natl Acad Sci U S A. 2006 Nov 21;103(47):17955-60. doi: 10.1073/pnas.0606212103. Epub 2006 Nov 8. Proc Natl Acad Sci U S A. 2006. PMID: 17093049 Free PMC article.
-
Presynaptic loss of dynamin-related protein 1 impairs synaptic vesicle release and recycling at the mouse calyx of Held.J Physiol. 2018 Dec;596(24):6263-6287. doi: 10.1113/JP276424. Epub 2018 Nov 10. J Physiol. 2018. PMID: 30285293 Free PMC article.
-
Dynamin Superfamily at Pre- and Postsynapses: Master Regulators of Synaptic Transmission and Plasticity in Health and Disease.Neuroscientist. 2022 Feb;28(1):41-58. doi: 10.1177/1073858420974313. Epub 2020 Dec 10. Neuroscientist. 2022. PMID: 33300419 Review.
-
[Recent advances in the study of synaptic endocytosis key protein: Dynamin].Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2014 Oct;39(10):1088-92. doi: 10.11817/j.issn.1672-7347.2014.10.018. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2014. PMID: 25355262 Review. Chinese.
Cited by
-
CUL3 Deficiency Causes Social Deficits and Anxiety-like Behaviors by Impairing Excitation-Inhibition Balance through the Promotion of Cap-Dependent Translation.Neuron. 2020 Feb 5;105(3):475-490.e6. doi: 10.1016/j.neuron.2019.10.035. Epub 2019 Nov 25. Neuron. 2020. PMID: 31780330 Free PMC article.
-
Myosin II regulates activity dependent compensatory endocytosis at central synapses.J Neurosci. 2013 Oct 9;33(41):16131-45. doi: 10.1523/JNEUROSCI.2229-13.2013. J Neurosci. 2013. PMID: 24107946 Free PMC article.
-
Ubiquitin-Synaptobrevin Fusion Protein Causes Degeneration of Presynaptic Motor Terminals in Mice.J Neurosci. 2015 Aug 19;35(33):11514-31. doi: 10.1523/JNEUROSCI.5288-14.2015. J Neurosci. 2015. PMID: 26290230 Free PMC article.
-
Effective knockdown-replace gene therapy in a novel mouse model of DNM1 developmental and epileptic encephalopathy.Mol Ther. 2024 Oct 2;32(10):3318-3330. doi: 10.1016/j.ymthe.2024.08.009. Epub 2024 Aug 10. Mol Ther. 2024. PMID: 39127888 Free PMC article.
-
Presynaptic ultrastructural plasticity along CA3→CA1 axons during long-term potentiation in mature hippocampus.J Comp Neurol. 2013 Dec 1;521(17):3898-912. doi: 10.1002/cne.23384. J Comp Neurol. 2013. PMID: 23784793 Free PMC article.
References
-
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. - PubMed
-
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. - PubMed
-
- Schneggenburger R, Sakaba T, Neher E. Vesicle pools and short-term synaptic depression: Lessons from a large synapse. Trends Neurosci. 2002;25:206–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

