Comparison of oxidative stress and inflammation induced by different intravenous iron sucrose similar preparations in a rat model
- PMID: 22309085
- PMCID: PMC3343386
- DOI: 10.2174/187152812798889358
Comparison of oxidative stress and inflammation induced by different intravenous iron sucrose similar preparations in a rat model
Abstract
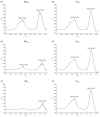
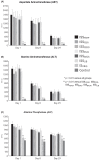
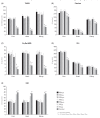
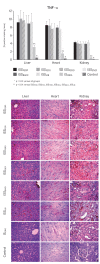
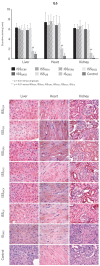
Iron sucrose originator (IS(ORIG)) has been used to treat iron deficiency and iron deficiency anemia for decades. Iron sucrose similars (ISSs) have recently entered the market. In this non-clinical study of non-anemic rats, five doses (40 mg iron/kg body weight) of six ISSs marketed in Asian countries, IS(ORIG) or saline solution (control) were administered intravenously over four weeks to compare their toxicologic effects. Vasodilatory effects, impaired renal function and hepatic damage were only observed in the ISS groups. Significantly elevated serum iron and transferrin saturation levels were observed in the ISS groups suggesting a higher release of iron resulting in higher amounts of non-transferrin bound (free) iron compared to IS(ORIG). This might explain the elevated oxidative stress and increased levels of inflammatory markers and antioxidant enzymes in the liver, heart and kidneys of ISS-treated animals. Physico-chemical analyses showed that the molecular structure of most of the ISSs differed greatly from that of the IS(ORIG). These differences may be responsible for the organ damage and oxidative stress observed in the ISS groups. Significant differences were also found between different lots of a single ISS product. In contrast, polarographic analyses of three different IS(ORIG) lots were identical, indicating that the molecular structure and thus the manufacturing process for IS(ORIG) is highly consistent. Data from this study suggest that ISSs and IS(ORIG) differ significantly. Therefore, before widespread use of these products it would be prudent to evaluate additional non-clinical and/or clinical data proving the safety, therapeutic equivalence and interchangeability of ISSs with IS(ORIG).
© 2012 Bentham Science Publishers
Figures
Similar articles
-
Nitrosative stress and apoptosis in non-anemic healthy rats induced by intravenous iron sucrose similars versus iron sucrose originator.Biometals. 2015 Apr;28(2):279-92. doi: 10.1007/s10534-015-9822-3. Epub 2015 Jan 22. Biometals. 2015. PMID: 25609135
-
Intravenous iron sucrose reverses anemia-induced cardiac remodeling, prevents myocardial fibrosis, and improves cardiac function by attenuating oxidative/nitrosative stress and inflammation.Int J Cardiol. 2016 Jun 1;212:84-91. doi: 10.1016/j.ijcard.2016.03.039. Epub 2016 Mar 17. Int J Cardiol. 2016. PMID: 27038710
-
Biodistribution and predictive hepatic gene expression of intravenous iron sucrose.J Pharmacol Toxicol Methods. 2013 Nov-Dec;68(3):374-83. doi: 10.1016/j.vascn.2013.04.005. Epub 2013 Apr 25. J Pharmacol Toxicol Methods. 2013. PMID: 23624021
-
Acute injury with intravenous iron and concerns regarding long-term safety.Clin J Am Soc Nephrol. 2006 Sep;1 Suppl 1:S19-23. doi: 10.2215/CJN.01420406. Clin J Am Soc Nephrol. 2006. PMID: 17699372 Review.
-
[Indications and practical management of parenteral iron therapy].Wien Klin Wochenschr. 2003 Jun 24;115(11):380-4. doi: 10.1007/BF03040356. Wien Klin Wochenschr. 2003. PMID: 12879735 Review. German.
Cited by
-
Intravenous Iron-Carbohydrate Nanoparticles and Their Similars. What Do We Choose?Maedica (Bucur). 2022 Jun;17(2):436-448. doi: 10.26574/maedica.2022.17.2.436. Maedica (Bucur). 2022. PMID: 36032600 Free PMC article.
-
Maximal standard dose of parenteral iron for hemodialysis patients: an MRI-based decision tree learning analysis.PLoS One. 2014 Dec 15;9(12):e115096. doi: 10.1371/journal.pone.0115096. eCollection 2014. PLoS One. 2014. PMID: 25506921 Free PMC article.
-
Markers of oxidative/nitrosative stress and inflammation in lung tissue of rats exposed to different intravenous iron compounds.Drug Des Devel Ther. 2017 Aug 1;11:2251-2263. doi: 10.2147/DDDT.S132612. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28814833 Free PMC article.
-
Safety of High-Dose Intravenous Iron in Hemodialysis Patients: Results from the National Health Insurance Service (2019-2020) in South Korea.J Clin Med. 2024 Dec 26;14(1):63. doi: 10.3390/jcm14010063. J Clin Med. 2024. PMID: 39797146 Free PMC article.
-
Comparative Evaluation of U.S. Brand and Generic Intravenous Sodium Ferric Gluconate Complex in Sucrose Injection: Physicochemical Characterization.Nanomaterials (Basel). 2018 Jan 5;8(1):25. doi: 10.3390/nano8010025. Nanomaterials (Basel). 2018. PMID: 29303999 Free PMC article.
References
-
- de Benoist B, McLean E, Egli I, Cogswell M. Worldwide prevalence of anaemia 1993-2005: WHO global database of anaemia. Geneva: World Health Organisation; 2008.
-
- Crichton R, Danielson B, Geisser P. Iron therapy With Special Emphasis on Intravenous Administration. 4th ed. Bremen: UNI-MED Verlag AG; 2008.
-
- Huch R, Schaefer R. Iron Deficiency and Iron Deficiency Anaemia. New York: Thieme Medical Publishers; 2006.
-
- Afzali B, Goldsmith D J. Intravenous iron therapy in renal failure: friend and foe? J. Nephrol. 2004;17:487–495. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

