Enantioselective construction of pyrrolidines by palladium-catalyzed asymmetric [3 + 2] cycloaddition of trimethylenemethane with imines
- PMID: 22309214
- PMCID: PMC3407810
- DOI: 10.1021/ja210981a
Enantioselective construction of pyrrolidines by palladium-catalyzed asymmetric [3 + 2] cycloaddition of trimethylenemethane with imines
Abstract
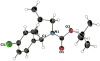
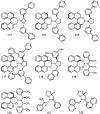
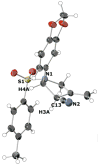
A protocol for the enantioselective [3 + 2] cycloaddition of trimethylenemethane (TMM) with imines has been developed. Central to this effort were the novel phosphoramidite ligands developed in our laboratories. The conditions developed to effect an asymmetric TMM reaction using 2-trimethylsilylmethyl allyl acetate were shown to be tolerant of a wide variety of imine acceptors to provide the corresponding pyrrolidine cycloadducts with excellent yields and selectivities. Use of a bis-2-naphthyl phosphoramidite allowed the successful cycloaddition of the parent TMM with N-Boc imines, and has further permitted the reaction of substituted donors with N-tosyl aldimines and ketimines in high regio-, diastereo-, and enantioselectivity. Use of a diphenylazetidine ligand allows the complementary synthesis of the exocyclic nitrile product shown, and we demonstrate control of the regioselectivity of the product based on manipulation of the reaction parameters.
Figures



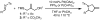
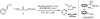


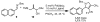

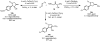



References
-
- Willstatter R. Annalen. 1903;317:204.
- Robinson R. J Chem Soc Trans. 1917;111:762.
- Humphrey AJ, O'Hagan D. Nat Prod Rep. 2001;18:494. - PubMed
-
-
Some selected examples of classical pyrrolidine syntheses include Wojcik B, Adkins H. J Am Chem Soc. 1934;56:2419–2424.
- Karrer P, Portmann P. Helv Chim Acta. 1948;31:2088–2092. - PubMed
- Dolfini JE, Dolfini DM. Tetrahedron Lett. 1965:2053–2058. - PubMed
- Basha FZ, DeBernardis JF. Tetrahedron Lett. 1984;25:5271–5274.
- Jiang C, Frontier AJ. Org Lett. 2007;9:4939–4942. - PubMed
- Lorthiois E, Marek I, Normant JF. J Org Chem. 1998;63:2442–2450. - PubMed
- Overman LE, Kakimoto MA. J Am Chem Soc. 1979;101:1310.
-
-
- Fache F, Schulz E, Tommasino ML, Lemaire M. Chem Rev. 2000;100:2159. - PubMed
-
- Dalko PI, Moisan L. Angew Chem Int Ed. 2004;43:5138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

