Detection of MMP-2 and MMP-9 activity in vivo with a triple-helical peptide optical probe
- PMID: 22309692
- PMCID: PMC3310325
- DOI: 10.1021/bc300027y
Detection of MMP-2 and MMP-9 activity in vivo with a triple-helical peptide optical probe
Abstract
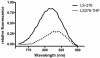
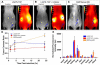
We report a novel activatable NIR fluorescent probe for in vivo detection of cancer-related matrix metalloproteinase (MMP) activity. The probe is based on a triple-helical peptide substrate (THP) with high specificity for MMP-2 and MMP-9 relative to other members of the MMP family. MMP-2 and MMP-9 (also known as gelatinases) are specifically associated with cancer cell invasion and cancer-related angiogenesis. At the center of each 5 kDa peptide strand is a gelatinase sensitive sequence flanked by 2 Lys residues conjugated with NIR fluorescent dyes. Upon self-assembly of the triple-helical structure, the 3 peptide chains intertwine, bringing the fluorophores into close proximity and reducing fluorescence via quenching. Upon enzymatic cleavage of the triple-helical peptide, 6 labeled peptide chains are released, resulting in an amplified fluorescent signal. The fluorescence yield of the probe increases 3.8-fold upon activation. Kinetic analysis showed a rate of LS276-THP hydrolysis by MMP-2 (k(cat)/K(M) = 30,000 s(-1) M(-1)) similar to that of MMP-2 catalysis of an analogous fluorogenic THP. Administration of LS276-THP to mice bearing a human fibrosarcoma xenografted tumor resulted in a tumor fluorescence signal more than 5-fold greater than that of muscle. This signal enhancement was reduced by treatment with the MMP inhibitor Ilomostat, indicating that the observed tumor fluorescence was indeed enzyme mediated. These results are the first to demonstrate that triple-helical peptides are suitable for highly specific in vivo detection of tumor-related MMP-2 and MMP-9 activity.
Figures
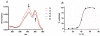

References
-
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. - PubMed
-
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. - PubMed
-
- Pacheco MM, Mourao M, Mantovani EB, Nishimoto IN, Brentani MM. Expression of gelatinases A and B, stromelysin-3 and matrilysin genes in breast carcinomas: clinico-pathological correlations. Clin Exp Metastasis. 1998;16:577–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

