Allosteric regulation of substrate channeling and catalysis in the tryptophan synthase bienzyme complex
- PMID: 22310642
- PMCID: PMC3702454
- DOI: 10.1016/j.abb.2012.01.016
Allosteric regulation of substrate channeling and catalysis in the tryptophan synthase bienzyme complex
Abstract
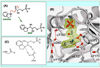
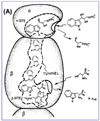
The tryptophan synthase α2β2 bi-enzyme complex catalyzes the last two steps in the synthesis of l-tryptophan (l-Trp). The α-subunit catalyzes cleavage of 3-indole-d-glycerol 3'-phosphate (IGP) to give indole and d-glyceraldehyde 3'-phosphate (G3P). Indole is then transferred (channeled) via an interconnecting 25Å-long tunnel, from the α-subunit to the β-subunit where it reacts with l-Ser in a pyridoxal 5'-phosphate-dependent reaction to give l-Trp and a water molecule. The efficient utilization of IGP and l-Ser by tryptophan synthase to synthesize l-Trp utilizes a system of allosteric interactions that (1) function to switch the α-site on and off at different stages of the β-subunit catalytic cycle, and (2) prevent the escape of the channeled intermediate, indole, from the confines of the α- and β-catalytic sites and the interconnecting tunnel. This review discusses in detail the chemical origins of the allosteric interactions responsible both for switching the α-site on and off, and for triggering the conformational changes between open and closed states which prevent the escape of indole from the bienzyme complex.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures















References
-
- Bertrand K, Korn L, Lee F, Platt T, Squires CL, Squires C, Yanofsky C. New features of the regulation of the tryptophan operon. Science. 1975;189:22–26. - PubMed
-
- Yanofsky C, Crawford IP. In: Tryptophan synthase. 3rd ed. Boyer PD, editor. New York: The Enzymes, Academic Press; 1972. pp. 1–31.
-
- Miles EW. Tryptophan synthase: structure, function, and subunit interaction. Adv. Enzymol. Relat Areas Mol. Biol. 1979;49:127–186. - PubMed
-
- Umbreit W, Wood W, Gunsalus I. The activity of pyridoxal phosphate in tryptophan formation by cell-free enzyme preparations. J. Biol. Chem. 1946;165:731–732. - PubMed
-
- Yanofsky C. Tryptophan synthetase from Neutrospora. In: Colowick SP, Kaplan NO, editors. Methods of Enzymol. vol. II. N. Y.: Academic Press, Inc.; 1995. pp. 233–238.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

