SIRT1 modulates MAPK pathways in ischemic-reperfused cardiomyocytes
- PMID: 22311064
- PMCID: PMC11114949
- DOI: 10.1007/s00018-012-0925-5
SIRT1 modulates MAPK pathways in ischemic-reperfused cardiomyocytes
Abstract
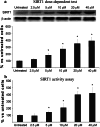
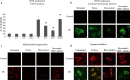
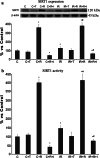
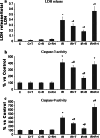
SIRT1, an ubiquitous NAD(+)-dependent deacetylase that plays a role in biological processes such as longevity and stress response, is significantly activated in response to reactive oxygen species (ROS) production. Resveratrol (Resv), an important activator of SIRT1, has been shown to exert major health benefits in diseases associated with oxidative stress. In ischemia-reperfusion (IR) injury, a major role has been attributed to the mitogen-activated protein kinase (MAPK) pathway, which is upregulated in response to a variety of stress stimuli, including oxidative stress. In neonatal rat ventricular cardiomyocytes subjected to simulated IR, the effect of Resv-induced SIRT1 activation and the relationships with the MAPK pathway were investigated. Resv-induced SIRT1 overexpression protected cardiomyocytes from oxidative injury, mitochondrial dysfunction, and cell death induced by IR. For the first time, we demonstrate that SIRT1 overexpression positively affects the MAPK pathway-via Akt/ASK1 signaling-by reducing p38 and JNK phosphorylation and increasing ERK phosphorylation. These results reveal a new protective mechanism elicited by Resv-induced SIRT1 activation in IR tissues and suggest novel potential therapeutic targets to manage IR-induced cardiac dysfunction.
Figures
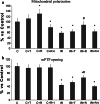
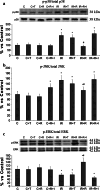


References
-
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. - PubMed
-
- Jeong J, Juhn K, Lee H, Kim SH, Min BH, Lee KM, Cho MH, Park GH, Lee KH. SIRT1 promotes DNA repair activity and deacetylation of Ku70. Exp Mol Med. 2007;39:8–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

