Expression, immunogenicity, histopathology, and potency of a mosquito-based malaria transmission-blocking recombinant vaccine
- PMID: 22311924
- PMCID: PMC3318422
- DOI: 10.1128/IAI.06212-11
Expression, immunogenicity, histopathology, and potency of a mosquito-based malaria transmission-blocking recombinant vaccine
Abstract
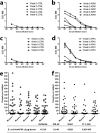
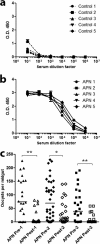
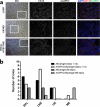
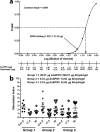
Vaccines have been at the forefront of global research efforts to combat malaria, yet despite several vaccine candidates, this goal has yet to be realized. A potentially effective approach to disrupting the spread of malaria is the use of transmission-blocking vaccines (TBV), which prevent the development of malarial parasites within their mosquito vector, thereby abrogating the cascade of secondary infections in humans. Since malaria is transmitted to human hosts by the bite of an obligate insect vector, mosquito species in the genus Anopheles, targeting mosquito midgut antigens that serve as ligands for Plasmodium parasites represents a promising approach to breaking the transmission cycle. The midgut-specific anopheline alanyl aminopeptidase N (AnAPN1) is highly conserved across Anopheles vectors and is a putative ligand for Plasmodium ookinete invasion. We have developed a scalable, high-yield Escherichia coli expression and purification platform for the recombinant AnAPN1 TBV antigen and report on its marked vaccine potency and immunogenicity, its capacity for eliciting transmission-blocking antibodies, and its apparent lack of immunization-associated histopathologies in a small-animal model.
Figures

Similar articles
-
Immunopotentiation by Lymph-Node Targeting of a Malaria Transmission-Blocking Nanovaccine.Front Immunol. 2021 Aug 27;12:729086. doi: 10.3389/fimmu.2021.729086. eCollection 2021. Front Immunol. 2021. PMID: 34512663 Free PMC article.
-
The use of transgenic Plasmodium berghei expressing the Plasmodium vivax antigen P25 to determine the transmission-blocking activity of sera from malaria vaccine trials.Vaccine. 2007 Jan 15;25(5):886-94. doi: 10.1016/j.vaccine.2006.09.035. Epub 2006 Sep 20. Vaccine. 2007. PMID: 17049690
-
The Anopheles-midgut APN1 structure reveals a new malaria transmission-blocking vaccine epitope.Nat Struct Mol Biol. 2015 Jul;22(7):532-9. doi: 10.1038/nsmb.3048. Epub 2015 Jun 15. Nat Struct Mol Biol. 2015. PMID: 26075520 Free PMC article.
-
Flipping the paradigm on malaria transmission-blocking vaccines.Trends Parasitol. 2008 Aug;24(8):364-70. doi: 10.1016/j.pt.2008.05.002. Epub 2008 Jul 1. Trends Parasitol. 2008. PMID: 18599352 Free PMC article. Review.
-
Development of malaria vaccines that block transmission of parasites by mosquito vectors.J Med Invest. 2002 Aug;49(3-4):118-23. J Med Invest. 2002. PMID: 12323000 Review.
Cited by
-
Historical Perspective and Biotechnological Trends to Block Arboviruses Transmission by Controlling Aedes aegypti Mosquitos Using Different Approaches.Front Med (Lausanne). 2020 Jun 23;7:275. doi: 10.3389/fmed.2020.00275. eCollection 2020. Front Med (Lausanne). 2020. PMID: 32656216 Free PMC article. Review.
-
Molecular interactions between parasite and mosquito during midgut invasion as targets to block malaria transmission.NPJ Vaccines. 2021 Nov 29;6(1):140. doi: 10.1038/s41541-021-00401-9. NPJ Vaccines. 2021. PMID: 34845210 Free PMC article. Review.
-
Metazoan Parasite Vaccines: Present Status and Future Prospects.Front Cell Infect Microbiol. 2018 Mar 13;8:67. doi: 10.3389/fcimb.2018.00067. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29594064 Free PMC article. Review.
-
Leishmania development in sand flies: parasite-vector interactions overview.Parasit Vectors. 2012 Dec 3;5:276. doi: 10.1186/1756-3305-5-276. Parasit Vectors. 2012. PMID: 23206339 Free PMC article. Review.
-
Transmission-Blocking Vaccines: Old Friends and New Prospects.Infect Immun. 2019 May 21;87(6):e00775-18. doi: 10.1128/IAI.00775-18. Print 2019 Jun. Infect Immun. 2019. PMID: 30962400 Free PMC article. Review.
References
-
- Abdulla S, et al. 2008. Safety and immunogenicity of RTS,S/AS02D malaria vaccine in infants. N. Engl. J. Med. 359:2533–2544 doi:10.1056/NEJMoa0807773 - DOI - PubMed
-
- Aide P, et al. 2011. Four year immunogenicity of the RTS,S/AS02(A) malaria vaccine in Mozambican children during a phase IIb trial. Vaccine 29:6059–6067 doi:10.1016/j.vaccine.2011.03.041 - DOI - PubMed
-
- Alonso PL, et al. 2011. A research agenda to underpin malaria eradication. PLoS Med. 8:e1000406 doi:10.1371/journal.pmed.1000406 - DOI - PMC - PubMed
-
- Angov E, Hillier CJ, Kincaid RL, Lyon JA. 2008. Heterologous protein expression is enhanced by harmonizing the codon usage frequencies of the target gene with those of the expression host. PLoS One 3:e2189 doi:10.1371/journal.pone.0002189 - DOI - PMC - PubMed
-
- Aponte JJ, et al. 2007. Safety of the RTS,S/AS02D candidate malaria vaccine in infants living in a highly endemic area of Mozambique: a double blind randomised controlled phase I/IIb trial. Lancet 370:1543–1551 doi:10.1016/S0140-6736(07)61542-6 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

