Campylobacter jejuni disrupts protective Toll-like receptor 9 signaling in colonic epithelial cells and increases the severity of dextran sulfate sodium-induced colitis in mice
- PMID: 22311925
- PMCID: PMC3318425
- DOI: 10.1128/IAI.06066-11
Campylobacter jejuni disrupts protective Toll-like receptor 9 signaling in colonic epithelial cells and increases the severity of dextran sulfate sodium-induced colitis in mice
Abstract
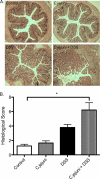
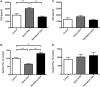
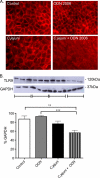
Inflammatory bowel disease (IBD) is characterized by chronic intestinal inflammation associated with a dysregulated immune response to commensal bacteria in susceptible individuals. The relapse of IBD may occur following an infection with Campylobacter jejuni. Apical epithelial Toll-like receptor 9 (TLR9) activation by bacterial DNA is reported to maintain colonic homeostasis. We investigated whether a prior C. jejuni infection disrupts epithelial TLR9 signaling and increases the severity of disease in a model of mild dextran sulfate sodium (DSS) colitis in mice. In a further attempt to identify mechanisms, T84 monolayers were treated with C. jejuni followed by a TLR9 agonist. Transepithelial resistance (TER) and dextran flux across confluent monolayers were monitored. Immunohistochemistry, Western blotting, and flow cytometry were used to examine TLR9 expression. Mice colonized by C. jejuni lacked any detectable pathology; however, in response to low levels of DSS, mice previously exposed to C. jejuni exhibited significantly reduced weight gain and increased occult blood and histological damage scores. Infected mice treated with DSS also demonstrated a significant reduction in levels of the anti-inflammatory cytokine interleukin-25. In vitro studies indicated that apical application of a TLR9 agonist enhances intestinal epithelial barrier function and that this response is lost in C. jejuni-infected monolayers. Furthermore, infected cells secreted significantly more CXCL8 following the basolateral application of a TLR9 agonist. Surface TLR9 expression was reduced in C. jejuni-infected monolayers subsequently exposed to a TLR9 agonist. In conclusion, infection by C. jejuni disrupts TLR9-induced reinforcement of the intestinal epithelial barrier, and colonization by C. jejuni increases the severity of mild DSS colitis.
Figures

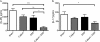


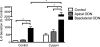
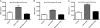
Similar articles
-
Effect of natural commensal-origin DNA on toll-like receptor 9 (TLR9) signaling cascade, chemokine IL-8 expression, and barrier integritiy of polarized intestinal epithelial cells.Inflamm Bowel Dis. 2010 Mar;16(3):410-27. doi: 10.1002/ibd.21057. Inflamm Bowel Dis. 2010. PMID: 19714766
-
Campylobacter jejuni mediated disruption of polarized epithelial monolayers is cell-type specific, time dependent, and correlates with bacterial invasion.Pediatr Res. 2008 Dec;64(6):599-604. doi: 10.1203/PDR.0b013e31818702b9. Pediatr Res. 2008. PMID: 18679160
-
Campylobacter jejuni induces colitis through activation of mammalian target of rapamycin signaling.Gastroenterology. 2012 Jan;142(1):86-95.e5. doi: 10.1053/j.gastro.2011.09.042. Epub 2011 Oct 1. Gastroenterology. 2012. PMID: 21963787 Free PMC article.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Epithelial toll-like receptor 9 signaling in colorectal inflammation and cancer: clinico-pathogenic aspects.World J Gastroenterol. 2013 Jul 14;19(26):4119-26. doi: 10.3748/wjg.v19.i26.4119. World J Gastroenterol. 2013. PMID: 23864774 Free PMC article. Review.
Cited by
-
Risk factors and clinical implication of superimposed Campylobacter jejuni infection in patients with underlying ulcerative colitis.Gastroenterol Rep (Oxf). 2016 Nov;4(4):287-292. doi: 10.1093/gastro/gov029. Epub 2015 Jul 8. Gastroenterol Rep (Oxf). 2016. PMID: 26159630 Free PMC article.
-
Source attribution of human Campylobacter isolates by MLST and fla-typing and association of genotypes with quinolone resistance.PLoS One. 2013 Nov 14;8(11):e81796. doi: 10.1371/journal.pone.0081796. eCollection 2013. PLoS One. 2013. PMID: 24244747 Free PMC article.
-
Multifunctional role of dextran sulfate sodium for in vivo modeling of intestinal diseases.BMC Immunol. 2012 Aug 1;13:41. doi: 10.1186/1471-2172-13-41. BMC Immunol. 2012. PMID: 22853702 Free PMC article.
-
Regulation of Intestinal Immune Responses through TLR Activation: Implications for Pro- and Prebiotics.Front Immunol. 2014 Feb 18;5:60. doi: 10.3389/fimmu.2014.00060. eCollection 2014. Front Immunol. 2014. PMID: 24600450 Free PMC article. Review.
-
The TLR9 agonist MGN1703 triggers a potent type I interferon response in the sigmoid colon.Mucosal Immunol. 2018 Mar;11(2):449-461. doi: 10.1038/mi.2017.59. Epub 2017 Aug 2. Mucosal Immunol. 2018. PMID: 28766555 Free PMC article. Clinical Trial.
References
-
- Bleich A, et al. 2009. CpG motifs of bacterial DNA exert protective effects in mouse models of IBD by antigen-independent tolerance induction. Gastroenterology 136:278–287 - PubMed
-
- Cario E. 2008. Barrier-protective function of intestinal epithelial Toll-like receptor 2. Mucosal Immunol. 1(Suppl 1):S62–S66 - PubMed
-
- Cario E, Gerken G, Podolsky DK. 2007. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132:1359–1374 - PubMed
-
- Cario E, Gerken G, Podolsky DK. 2004. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 127:224–238 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

