Electronic cigarettes: effective nicotine delivery after acute administration
- PMID: 22311962
- PMCID: PMC3524053
- DOI: 10.1093/ntr/ntr316
Electronic cigarettes: effective nicotine delivery after acute administration
Abstract
Introduction: Electronic cigarettes (ECs) are marketed as nicotine delivery devices. Two studies with EC-naïve participants suggest that ECs deliver little or no nicotine. In those studies, standard-sized ECs were used, though experienced EC users often use larger devices that house higher voltage and/or longer lasting batteries. Whether user experience and device characteristics influence EC nicotine delivery is uncertain. The purpose of the present study was to examine the effects of ECs in experienced users who were using their preferred devices.
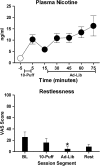
Methods: Eight EC users (3 women) who had been using ECs for at least 3 months, completed one 5-hr session using devices they provided and the flavor/strength nicotine cartridges they selected. Sessions consisted of 4 phases: baseline, 10 puffs (30-s interpuff interval) from the device, 1-hr ad lib puffing period, and a 2-hr rest period (no puffing). Outcome measures in each phase included plasma nicotine concentration, heart rate, and subjective ratings of nicotine/product effects and abstinence symptoms.
Results: Relative to baseline, plasma nicotine and heart rate increased significantly within 5 min of the first puff and remained elevated throughout the ad lib puffing period. Increases in ratings of direct effects of nicotine and product were observed as well as decreases in abstinence symptoms.
Conclusions: User experience and/or device characteristics likely influence EC nicotine delivery and other effects. Systematic manipulation of these and other variables could elucidate conditions that produce intended effects.
Figures
References
-
- Ayers JW, Ribisi KM, Brownstein JS. Tracking the rise in popularity of electronic nicotine delivery systems (electronic cigarettes) using search query surveillance. American Journal of Preventive Medicine. 2011;40:448–453. doi:10.1016/j.amepre.2010.12.007. - PubMed
-
- Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine & Tobacco Research. 2006;8:727–738. doi:10.1080/14622200600789585. - PubMed
-
- Bullen C, McRobie H, Thornley S, Glover M, Laugesen M. Effect of an electronic cigarette on desire to smoke and withdrawal, user preferences and nicotine delivery: Randomized cross-over trial. Tobacco Control. 2010;19:98–103. doi:10.1136/tc.2009.031567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

