A critical role for muscle ring finger-1 in acute lung injury-associated skeletal muscle wasting
- PMID: 22312013
- PMCID: PMC3360571
- DOI: 10.1164/rccm.201106-1150OC
A critical role for muscle ring finger-1 in acute lung injury-associated skeletal muscle wasting
Abstract
Rationale: Acute lung injury (ALI) is a debilitating condition associated with severe skeletal muscle weakness that persists in humans long after lung injury has resolved. The molecular mechanisms underlying this condition are unknown.
Objectives: To identify the muscle-specific molecular mechanisms responsible for muscle wasting in a mouse model of ALI.
Methods: Changes in skeletal muscle weight, fiber size, in vivo contractile performance, and expression of mRNAs and proteins encoding muscle atrophy-associated genes for muscle ring finger-1 (MuRF1) and atrogin1 were measured. Genetic inactivation of MuRF1 or electroporation-mediated transduction of miRNA-based short hairpin RNAs targeting either MuRF1 or atrogin1 were used to identify their role in ALI-associated skeletal muscle wasting.
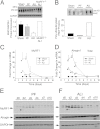
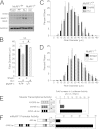
Measurements and main results: Mice with ALI developed profound muscle atrophy and preferential loss of muscle contractile proteins associated with reduced muscle function in vivo. Although mRNA expression of the muscle-specific ubiquitin ligases, MuRF1 and atrogin1, was increased in ALI mice, only MuRF1 protein levels were up-regulated. Consistent with these changes, suppression of MuRF1 by genetic or biochemical approaches prevented muscle fiber atrophy, whereas suppression of atrogin1 expression was without effect. Despite resolution of lung injury and down-regulation of MuRF1 and atrogin1, force generation in ALI mice remained suppressed.
Conclusions: These data show that MuRF1 is responsible for mediating muscle atrophy that occurs during the period of active lung injury in ALI mice and that, as in humans, skeletal muscle dysfunction persists despite resolution of lung injury.
Figures





Comment in
-
Lord of the RING: ubiquitination and directed degradation of skeletal muscle in acute lung injury.Am J Respir Crit Care Med. 2012 Apr 15;185(8):795-6. doi: 10.1164/rccm.201202-0225ED. Am J Respir Crit Care Med. 2012. PMID: 22505748 No abstract available.
References
-
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349 - PubMed
-
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693 - PubMed
-
- Ali NA, O'Brien JM, Jr, Hoffmann SP, Phillips G, Garland A, Finley JC, Almoosa K, Hejal R, Wolf KM, Lemeshow S, et al. ; Midwest Critical Care Consortium Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med 2008;178:261–268 - PubMed
-
- Fletcher SN, Kennedy DD, Ghosh IR, Misra VP, Kiff K, Coakley JH, Hinds CJ. Persistent neuromuscular and neurophysiologic abnormalities in long-term survivors of prolonged critical illness. Crit Care Med 2003;31:1012–1016 - PubMed
-
- Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, et al. Canadian Critical Care Trials Group One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 2003;348:683–693 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

