Activation of mitochondrial biogenesis by heme oxygenase-1-mediated NF-E2-related factor-2 induction rescues mice from lethal Staphylococcus aureus sepsis
- PMID: 22312014
- PMCID: PMC3360573
- DOI: 10.1164/rccm.201106-1152OC
Activation of mitochondrial biogenesis by heme oxygenase-1-mediated NF-E2-related factor-2 induction rescues mice from lethal Staphylococcus aureus sepsis
Abstract
Rationale: Mitochondrial damage is an important component of multiple organ failure syndrome, a highly lethal complication of severe sepsis that lacks specific therapy. Mitochondrial quality control is regulated in part by the heme oxygenase-1 (HO-1; Hmox1) system through the redox-regulated NF-E2-related factor-2 (Nrf2) transcription factor, but its role in mitochondrial biogenesis in Staphylococcus aureus sepsis is unknown.
Objectives: To test the hypothesis that Nrf2-dependent up-regulation of the HO-1/carbon monoxide (CO) system would preserve mitochondrial biogenesis and rescue mice from lethal S. aureus sepsis.
Methods: A controlled murine S. aureus peritonitis model with and without inhaled CO was examined for HO-1 and Nrf2 regulation of mitochondrial biogenesis and the resolution of hepatic mitochondrial damage.
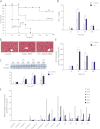
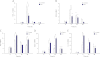
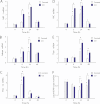
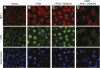
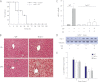
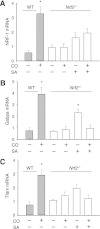
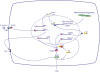
Measurements and main results: Sepsis survival was significantly enhanced using inhaled CO (250 ppm once-daily for 1 h), and linked mechanistically to Hmox1 induction and mitochondrial HO activity through Nrf2 transcriptional and Akt kinase activity. HO-1/CO stimulated Nrf2-dependent gene expression and nuclear accumulation of nuclear respiratory factor-1, -2α (Gabpa), and peroxisome proliferator-activated receptor gamma coactivator-1α; increased mitochondrial transcription factor-A and citrate synthase protein levels; and augmented mtDNA copy number. CO enhanced antiinflammatory IL-10 and reduced proinflammatory tumor necrosis factor-α production. By contrast, Nrf2(-/-) and Akt1(-/-) mice lacked CO induction of Hmox1 and mitochondrial biogenesis, and CO rescued neither strain from S. aureus sepsis.
Conclusions: We identify an inducible Nrf2/HO-1 regulatory cycle for mitochondrial biogenesis that is prosurvival and counter-inflammatory in sepsis, and describe targeted induction of mitochondrial biogenesis as a potential multiple organ failure therapy.
Figures



Comment in
-
Carbon monoxide, a modern "pharmakon" for sepsis.Am J Respir Crit Care Med. 2012 Apr 15;185(8):800-1. doi: 10.1164/rccm.201202-0224ED. Am J Respir Crit Care Med. 2012. PMID: 22505751 No abstract available.
References
-
- Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med 2007;35:1244–1250 - PubMed
-
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003;348:1546–1554 - PubMed
-
- Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002;360:219–223 - PubMed
-
- Vincent JL, Bruzzi de Carvalho F. Severity of illness. Semin Respir Crit Care Med 2010;31:31–38 - PubMed
-
- Bradley JR. TNF-mediated inflammatory disease. J Pathol 2008;214:149–160 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

