Virus-infected alveolar epithelial cells direct neutrophil chemotaxis and inhibit their apoptosis
- PMID: 22312020
- PMCID: PMC3380290
- DOI: 10.1165/rcmb.2011-0230OC
Virus-infected alveolar epithelial cells direct neutrophil chemotaxis and inhibit their apoptosis
Abstract
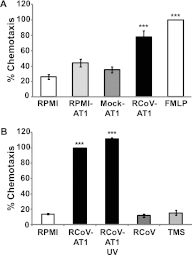
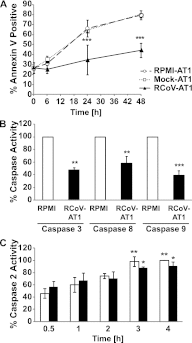
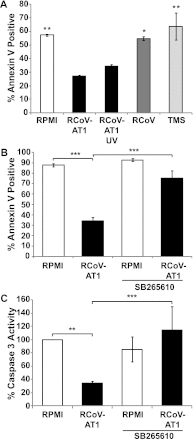
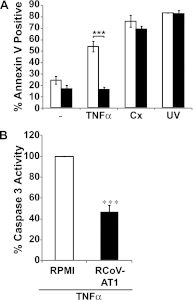
The alveolar epithelium is a critical target for pulmonary viruses and can produce proinflammatory cytokines and chemokines upon viral infection. However, the molecular interactions between virus-infected alveolar epithelial cells and inflammatory cells, including polymorphonuclear leukocytes (PMNs), have not been thoroughly characterized. Rat coronavirus (RCoV) is used as a model to study the immune response to viral infection in the lung of the natural host. We have developed an in vitro model to characterize the response of PMNs to RCoV-infected type I-like alveolar epithelial (AT1) cells, the primary target for RCoV infection in the alveoli. Multiple CXC chemokines that signal through CXCR2 were required for PMN chemotaxis toward medium from RCoV-infected AT1-like cells (RCoV-AT1). Furthermore, RCoV-AT1 inhibited spontaneous PMN apoptosis, including activation of effector caspase 3 and initiator caspases 8 and 9. Use of a selective inhibitor of CXCR2, SB265610, demonstrated that CXCR2 signaling was required for RCoV-AT1-mediated inhibition of PMN apoptosis. These data suggest that CXC chemokines produced by RCoV-infected AT1-like cells inhibit PMN apoptosis during infection. These studies provide new insight into the molecular mechanisms whereby alveolar epithelial cells direct the functions of PMNs during viral infection of the lung.
Figures


References
-
- Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol 2007;20:108–119 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

