Uptake and economic impact of first-cycle colony-stimulating factor use during adjuvant treatment of breast cancer
- PMID: 22312106
- PMCID: PMC3295569
- DOI: 10.1200/JCO.2011.37.7499
Uptake and economic impact of first-cycle colony-stimulating factor use during adjuvant treatment of breast cancer
Abstract
Purpose: In 2002, pegfilgrastim was approved by the US Food and Drug Administration and the benefits of dose-dense breast cancer chemotherapy, especially for hormone receptor (HR) -negative tumors, were reported. We examined first-cycle colony-stimulating factor use (FC-CSF) before and after 2002 and estimated US expenditures for dose-dense chemotherapy.
Methods: We identified patients in Surveillance, Epidemiology, and End Results-Medicare greater than 65 years old with stages I to III breast cancer who had greater than one chemotherapy claim within 6 months of diagnosis(1998 to 2005) and classified patients with an average cycle length less than 21 days as having received dose-dense chemotherapy. The associations of patient, tumor, and physician-related factors with the receipt of any colony-stimulating factor (CSF) and FC-CSF use were analyzed by using generalized estimating equations. CSF costs were estimated for patients who were undergoing dose-dense chemotherapy.
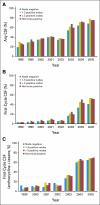
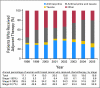
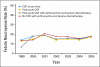
Results: Among the 10,773 patients identified, 5,266 patients (48.9%) had a CSF claim. CSF use was stable between 1998 and 2002 and increased from 36.8% to 73.7% between 2002 and 2005, FC-CSF use increased from 13.2% to 67.9%, and pegfilgrastim use increased from 4.1% to 83.6%. In a multivariable analysis, CSF use was associated with age and chemotherapy type and negatively associated with black/Hispanic race, rural residence, and shorter chemotherapy duration. FC-CSF use was associated with high socioeconomic status but not with age or race/ethnicity. The US annual CSF expenditure for women with HR-positive tumors treated with dose-dense chemotherapy is estimated to be $38.8 million.
Conclusion: A rapid increase in FC-CSF use occurred over a short period of time, which was likely a result of the reported benefits of dose-dense chemotherapy and the ease of pegfilgrastim administration. Because of the increasing evidence that elderly HR-positive patients do not benefit from dose-dense chemotherapy, limiting pegfilgrastim use would combat the increasing costs of cancer care.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Wiser use of dose-dense adjuvant therapy in breast cancer.J Clin Oncol. 2012 Mar 10;30(8):772-4. doi: 10.1200/JCO.2011.40.2537. Epub 2012 Feb 6. J Clin Oncol. 2012. PMID: 22312107 No abstract available.
-
Can we really use retrospective subset analyses and surveillance, epidemiology, and end results data to drive clinical practice?J Clin Oncol. 2012 Sep 1;30(25):3148-9; author reply 3149-50. doi: 10.1200/JCO.2012.43.4746. Epub 2012 Jul 30. J Clin Oncol. 2012. PMID: 22851555 No abstract available.
References
-
- Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. - PubMed
-
- Venturini M, Del Mastro L, Aitini E, et al. Dose-dense adjuvant chemotherapy in early breast cancer patients: Results from a randomized trial. J Natl Cancer Inst. 2005;97:1724–1733. - PubMed
-
- Baldini E, Gardin G, Giannessi PG, et al. Accelerated versus standard cyclophosphamide, epirubicin and 5-fluorouracil or cyclophosphamide, methotrexate and 5-fluorouracil: A randomized phase III trial in locally advanced breast cancer. Ann Oncol. 2003;14:227–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

