Self-reactive human CD4 T cell clones form unusual immunological synapses
- PMID: 22312112
- PMCID: PMC3280872
- DOI: 10.1084/jem.20111485
Self-reactive human CD4 T cell clones form unusual immunological synapses
Abstract
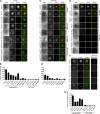
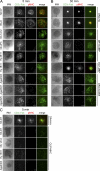
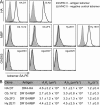
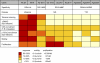
Recognition of self-peptide-MHC (pMHC) complexes by CD4 T cells plays an important role in the pathogenesis of many autoimmune diseases. We analyzed formation of immunological synapses (IS) in self-reactive T cell clones from patients with multiple sclerosis and type 1 diabetes. All self-reactive T cells contained a large number of phosphorylated T cell receptor (TCR) microclusters, indicative of active TCR signaling. However, they showed little or no visible pMHC accumulation or transport of TCR-pMHC complexes into a central supramolecular activation cluster (cSMAC). In contrast, influenza-specific T cells accumulated large quantities of pMHC complexes in microclusters and a cSMAC, even when presented with 100-fold lower pMHC densities. The self-reactive T cells also maintained a high degree of motility, again in sharp contrast to virus-specific T cells. 2D affinity measurements of three of these self-reactive T cell clones demonstrated a normal off-rate but a slow on-rate of TCR binding to pMHC. These unusual IS features may facilitate escape from negative selection by self-reactive T cells encountering very small amounts of self-antigen in the thymus. However, these same features may enable acquisition of effector functions by self-reactive T cells encountering large amounts of self-antigen in the target organ of the autoimmune disease.
Figures
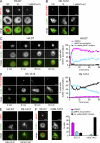
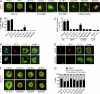

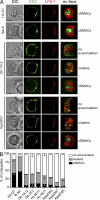
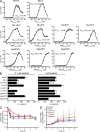

Comment in
-
Broken synapses in autoimmunity.Nat Rev Immunol. 2012 Feb 17;12(3):152. doi: 10.1038/nri3176. Nat Rev Immunol. 2012. PMID: 22343571 No abstract available.
References
-
- Anderson A.C., Nicholson L.B., Legge K.L., Turchin V., Zaghouani H., Kuchroo V.K. 2000. High frequency of autoreactive myelin proteolipid protein-specific T cells in the periphery of naive mice: mechanisms of selection of the self-reactive repertoire. J. Exp. Med. 191:761–770 10.1084/jem.191.5.761 - DOI - PMC - PubMed
-
- Barrett J.C., Clayton D.G., Concannon P., Akolkar B., Cooper J.D., Erlich H.A., Julier C., Morahan G., Nerup J., Nierras C., et al. ; Type 1 Diabetes Genetics Consortium 2009. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 41:703–707 10.1038/ng.381 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

