Association of influenza virus proteins with membrane rafts
- PMID: 22312341
- PMCID: PMC3265303
- DOI: 10.1155/2011/370606
Association of influenza virus proteins with membrane rafts
Abstract
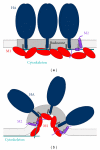
Assembly and budding of influenza virus proceeds in the viral budozone, a domain in the plasma membrane with characteristics of cholesterol/sphingolipid-rich membrane rafts. The viral transmembrane glycoproteins hemagglutinin (HA) and neuraminidase (NA) are intrinsically targeted to these domains, while M2 is seemingly targeted to the edge of the budozone. Virus assembly is orchestrated by the matrix protein M1, binding to all viral components and the membrane. Budding progresses by protein- and lipid-mediated membrane bending and particle scission probably mediated by M2. Here, we summarize the experimental evidence for this model with emphasis on the raft-targeting features of HA, NA, and M2 and review the functional importance of raft domains for viral protein transport, assembly and budding, environmental stability, and membrane fusion.
Figures

References
-
- Shishkov AV, Bogacheva EN, Dolgov AA, et al. The in situ structural characterization of the influenza A virus matrix M1 protein within a virion. Protein and Peptide Letters. 2009;16(11):1407–1413. - PubMed
-
- Whittaker GR. Intracellular trafficking of influenza virus: clinical implications for molecular medicine. Expert Reviews in Molecular Medicine. 2001;2001:1–13. - PubMed
-
- Veit M, Schmidt MFG. Palmitoylation of influenza virus proteins. Berliner und Münchener Tierärztliche Wochenschrift. 2006;119(3-4):112–122. - PubMed
-
- Garten W, Klenk HD. Understanding influenza virus pathogenicity. Trends in Microbiology. 1999;7(3):99–100. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

