MDA5 cooperatively forms dimers and ATP-sensitive filaments upon binding double-stranded RNA
- PMID: 22314235
- PMCID: PMC3321199
- DOI: 10.1038/emboj.2012.19
MDA5 cooperatively forms dimers and ATP-sensitive filaments upon binding double-stranded RNA
Abstract
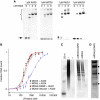
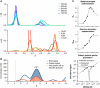
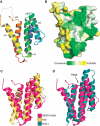
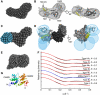
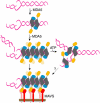
Melanoma differentiation-associated gene-5 (MDA5) detects viral double-stranded RNA in the cytoplasm. RNA binding induces MDA5 to activate the signalling adaptor MAVS through interactions between the caspase recruitment domains (CARDs) of the two proteins. The molecular mechanism of MDA5 signalling is not well understood. Here, we show that MDA5 cooperatively binds short RNA ligands as a dimer with a 16-18-basepair footprint. A crystal structure of the MDA5 helicase-insert domain demonstrates an evolutionary relationship with the archaeal Hef helicases. In X-ray solution structures, the CARDs in unliganded MDA5 are flexible, and RNA binds on one side of an asymmetric MDA5 dimer, bridging the two subunits. On longer RNA, full-length and CARD-deleted MDA5 constructs assemble into ATP-sensitive filaments. We propose a signalling model in which the CARDs on MDA5-RNA filaments nucleate the assembly of MAVS filaments with the same polymeric geometry.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Aida K, Nishida Y, Tanaka S, Maruyama T, Shimada A, Awata T, Suzuki M, Shimura H, Takizawa S, Ichijo M, Akiyama D, Furuya F, Kawaguchi A, Kaneshige M, Itakura J, Fujii H, Endo T, Kobayashi T (2011) RIG-I- and MDA5-initiated innate immunity linked with adaptive immunity accelerates beta-cell death in fulminant type 1 diabetes. Diabetes 60: 884–889 - PMC - PubMed
-
- Baum J, Tonkin CJ, Paul AS, Rug M, Smith BJ, Gould SB, Richard D, Pollard TD, Cowman AF (2008) A malaria parasite formin regulates actin polymerization and localizes to the parasite-erythrocyte moving junction during invasion. Cell Host Microbe 3: 188–198 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

