Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design
- PMID: 22314428
- PMCID: PMC12261306
- DOI: 10.1016/j.jhep.2012.01.008
Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design
Abstract
Background & aims: Transarterial chemoembolisation (TACE) improves survival of properly selected patients with hepatocellular carcinoma (HCC). Drug eluting beads (DEB) provide a calibrated and homogenous procedure while increasing efficacy. Outcome data applying this technology is lacking, and this is instrumental for clinical decision-making and for trial design. We evaluated the survival of HCC patients treated with DEB-TACE following a strict selection (preserved liver function, absence of symptoms, extrahepatic spread or vascular invasion).
Methods: We registered baseline characteristics, the development of treatment-related adverse events, and the overall survival of all HCC patients treated by DEB-TACE from February 2004 to June 2010.
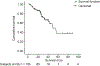
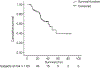
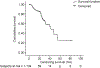
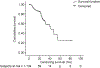
Results: One hundred and four patients were treated with DEB-TACE. All but one were cirrhotic, 62.5% HCV+, 95% Child-Pugh A, 41 BCLC-A and 63 BCLC-B. Causes of DEB-TACE treatment in BCLC-A patients were: 35 unfeasible ablation, and six post-treatment recurrences. After a median follow-up of 24.5 months, 38 patients had died, two patients had received transplantation and 24 had received sorafenib because of untreatable tumour progression. Median survival of the cohort was 48.6 months (95% CI: 36.9-61.2), while it was 54.2 months in BCLC stage A and 47.7 months in stage B. Median survival after censoring follow-up at time of transplant/sorafenib was 47.7 (95%CI: 37.9-57.5) months.
Conclusions: These data validate the safety of DEB-TACE and show that the survival expectancy applying current selection criteria and technique is better than that previously reported. A 50% survival at 4 years should be considered when suggesting treatment for patients fitting into controversial scenarios such as expanded criteria for transplantation/resection for multifocal HCC.
Copyright © 2012 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest
J. Bruix had exerted as consultant or received grants from: Sumitomo, Pharmexa, Eisai, Biocompatibles, Biolliance, Bayer Schering, Lilly, Novartis, Arqule, Angiodynamics, Kowa, Imclone.
C. Ayuso, M. Burrel, A. Forner, M. Reig, and C. Rodriguez-Lope had exerted as consultant: Terumo Europe, Bayer Schering.
M. Barrufet and M. Real had exerted as consultant: Terumo Europe.
M. Llovet received Research Support from: Bayer Pharmaceutical, Bristol Myers Squibb and Consultancy Agreements from Bayer Pharmaceutical, Bristol Myers Squibb, Imclone, Biocompatibles.
Figures
Comment in
-
Transarterial therapies for hepatocellular carcinoma (HCC): a long way towards standardization.J Hepatol. 2013 Jan;58(1):194. doi: 10.1016/j.jhep.2012.08.028. Epub 2012 Oct 6. J Hepatol. 2013. PMID: 23046673 No abstract available.
References
-
- Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol 2007;46:474–481. - PubMed
-
- Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol 2007;5:1100–1108. - PubMed
-
- Malagari K, Chatzimichael K, Alexopoulou E, Kelekis A, Hall B, Dourakis S, et al. Transarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patients. Cardiovasc Intervent Radiol 2008;31:269–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous