Exploiting the anti-HIV-1 activity of acyclovir: suppression of primary and drug-resistant HIV isolates and potentiation of the activity by ribavirin
- PMID: 22314523
- PMCID: PMC3346610
- DOI: 10.1128/AAC.05986-11
Exploiting the anti-HIV-1 activity of acyclovir: suppression of primary and drug-resistant HIV isolates and potentiation of the activity by ribavirin
Abstract
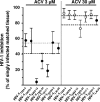
Multiple clinical trials have demonstrated that herpes simplex virus 2 (HSV-2) suppressive therapy using acyclovir (ACV) or valacyclovir in HIV-1/HSV-2-infected persons increased the patient's survival and decreased the HIV-1 load. It has been shown that the incorporation of ACV-monophosphate into the nascent DNA chain instead of dGMP results in the termination of viral DNA elongation and directly inhibits laboratory strains of HIV-1. We evaluated here the anti-HIV activity of ACV against primary HIV-1 isolates of different clades and coreceptor specificity and against viral isolates resistant to currently used drugs, including zidovudine, lamivudine, nevirapine, a combination of nucleoside reverse transcriptase inhibitors (NRTIs), a fusion inhibitor, and two protease inhibitors. We found that, at clinically relevant concentrations, ACV inhibits the replication of these isolates in human tissues infected ex vivo. Moreover, addition of ribavirin, an antiviral capable of depleting the pool of intracellular dGTP, potentiated the ACV-mediated HIV-1 suppression. These data warrant further clinical investigations of the benefits of using inexpensive and safe ACV alone or in combination with other drugs against HIV-1, especially to complement or delay highly active antiretroviral therapy (HAART) initiation in low-resource settings.
Figures


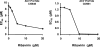
References
-
- Arion D, Kaushik N, McCormick S, Borkow G, Parniak MA. 1998. Phenotypic mechanism of HIV-1 resistance to 3′-azido-3′-deoxythymidine (AZT): increased polymerization processivity and enhanced sensitivity to pyrophosphate of the mutant viral reverse transcriptase. Biochemistry 37:15908–15917 - PubMed
-
- Balzarini J, Lee CK, Herdewijn P, De Clercq E. 1991. Mechanism of the potentiating effect of ribavirin on the activity of 2′,3′-dideoxyinosine against human immunodeficiency virus. J. Biol. Chem. 266:21509–21514 - PubMed
-
- Balzarini J, Naesens L, Robins MJ, De Clercq E. 1990. Potentiating effect of ribavirin on the in vitro and in vivo antiretrovirus activities of 2′,3′-dideoxyinosine and 2′,3′-dideoxy-2,6-diaminopurine riboside. J. Acquir. Immune Defic. Syndr. 3:1140–1147 - PubMed

