Product quality of parenteral vancomycin products in the United States
- PMID: 22314525
- PMCID: PMC3370807
- DOI: 10.1128/AAC.05344-11
Product quality of parenteral vancomycin products in the United States
Abstract
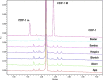
In response to concerns raised about the quality of parenteral vancomycin products, the U.S. Food and Drug Administration (FDA) is investigating the product quality of all FDA-approved parenteral vancomycin products available in the United States. Product quality was evaluated independently at two FDA Office of Testing and Research (FDA-OTR) sites. In the next phase of the investigation, being done in collaboration with the National Institute of Allergy and Infectious Diseases, the in vivo activity of these products will be evaluated in an appropriate animal model. This paper summarizes results of the FDA investigation completed thus far. One site used a validated ultrahigh-pressure liquid chromatography method (OTR-UPLC), and the second site used the high-performance liquid chromatography (HPLC) method for related substances provided in the British Pharmacopeia (BP) monograph for vancomycin intravenous infusion. Similar results were obtained by the two FDA-OTR laboratories using two different analytical methods. The products tested had 90 to 95% vancomycin B (active component of vancomycin) by the BP-HPLC method and 89 to 94% vancomycin by OTR-UPLC methods. Total impurities were 5 to 10% by BP-HPLC and 6 to 11% by OTR-UPLC methods. No single impurity was >2.0%, and the CDP-1 level was ≤ 2.0% across all products. Some variability in impurity profiles of the various products was observed. No adverse product quality issues were identified with the six U.S. vancomycin parenteral products. The quality parameters of all parenteral vancomycin products tested surpassed the United States Pharmacopeia acceptance criteria. Additional testing will characterize in vivo performance characteristics of these products.
Figures

References
-
- Code of Federal Regulations 2011. Title 21. Food and Drugs. Chapter I. Food and Drug Administration. Subchapter D. Drugs for human use. Part 314. Applications for FDA approval to market a new drug. 21 CFR 314.94(a)(3).
-
- Code of Federal Regulations 2011. Title 21. Food and Drugs. Chapter I. Food and Drug Administration. Subchapter D. Drugs for human use. Part 314. Applications for FDA approval to market a new drug. 21 CFR 314.94(a).
-
- Code of Federal Regulations 2011. Title 21. Food and Drugs. Chapter I. Food and Drug Administration. Subchapter D. Drugs for human use. Part 314. Applications for FDA approval to market a new drug. 21 CFR 314.94(a)(9)(iii).
-
- Code of Federal Regulations 2011. Title 21. Food and Drugs. Chapter I. Food and Drug Administration. Subchapter D. Drugs for human use. Part 314. Applications for FDA approval to market a new drug. 21 CFR 314.94(a)(9).
-
- Code of Federal Regulations 2011. Title 21. Food and Drugs. Chapter I. Food and Drug Administration. Subchapter D. Drugs for human use. Part 314. Applications for FDA approval to market a new drug. 21 CFR 314.94(a)(9)(i) [citing 21 CFR 314.50(d)(1)].
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

