Direct improvement of quality of life using a tailored quality of life diagnosis and therapy pathway: randomised trial in 200 women with breast cancer
- PMID: 22315052
- PMCID: PMC3305975
- DOI: 10.1038/bjc.2012.4
Direct improvement of quality of life using a tailored quality of life diagnosis and therapy pathway: randomised trial in 200 women with breast cancer
Abstract
Background: Despite thousands of papers, the value of quality of life (QoL) in curing disease remains uncertain. Until now, we lacked tools for the diagnosis and specific treatment of diseased QoL. We approached this problem stepwise by theory building, modelling, an exploratory trial and now a definitive randomised controlled trial (RCT) in breast cancer, whose results we report here.
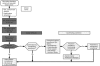
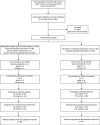
Methods: In all, 200 representative Bavarian primary breast cancer patients were recruited by five hospitals and treated by 146 care professionals. Patients were randomised to either (1) a novel care pathway including diagnosis of 'diseased' QoL (any QoL measure below 50 points) using a QoL profile and expert report sent to the patient's coordinating practitioner, who arranged QoL therapy consisting of up to five standardised treatments for specific QoL defects or (2) standard postoperative care adhering to the German national guideline for breast cancer. The primary end point was the proportion of patients in each group with diseased QoL 6 months after surgery. Patients were blinded to their allocated group.
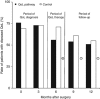
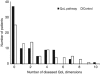
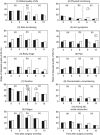
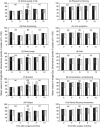
Results: At 0 and 3 months after surgery, diseased QoL was diagnosed in 70% of patients. The QoL pathway reduced rates of diseased QoL to 56% at 6 months, especially in emotion and coping, compared with 71% in controls (P=0.048). Relative risk reduction was 21% (95% confidence interval (CI): 0-37), absolute risk reduction 15% (95% CI: 0.3-29), number needed to treat (NNT)=7 (95% CI: 3-37). When QoL therapy finished after successful treatment, diseased QoL often returned again, indicating good responsiveness of the QoL pathway.
Conclusion: A three-component outcome system including clinician-derived objective, patient-reported subjective end points and qualitative analysis of clinical relevance was developed in the last 10 years for cancer as a complex intervention. A separate QoL pathway was implemented for the diagnosis and treatment of diseased QoL and its effectiveness tested in a community-based, pragmatic, definitive RCT. While the pathway was active, it was effective with an NNT of 7.
Figures


References
-
- Bauhofer A, Plaul U, Torossian A, Koller M, Stinner B, Celik I, Sitter H, Greger B, Middeke M, Schein M, Wyatt J, Nyström P, Hartung T, Rothmund M, Lorenz W (2007) Perioperative prophylaxis with granulocyte colony-stimulating factor (G-CSF) in high-risk colorectal cancer patients for an improved recovery: a randomized controlled trial. Surgery 141: 501–510 - PubMed
-
- Bauhofer A, Schwarting R, Köster M, Schmitt A, Lorenz W, Pawlak C (2004) Sickness behavior of rats with abdominal sepsis can be improved by antibiotic and G-CSF prophylaxis in clinic modeling randomized trials. Inflamm Res 53: 697–705 - PubMed
-
- Beliveau R, Gingras D (2005) Les Aliments Contre le Cancer. Editions du Trecarre Quebecor Media Inc: Outremont, QC Canada
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

