Germline TRAV5D-4 T-cell receptor sequence targets a primary insulin peptide of NOD mice
- PMID: 22315318
- PMCID: PMC3314349
- DOI: 10.2337/db11-1113
Germline TRAV5D-4 T-cell receptor sequence targets a primary insulin peptide of NOD mice
Abstract
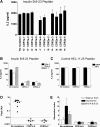
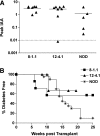
There is accumulating evidence that autoimmunity to insulin B chain peptide, amino acids 9-23 (insulin B:9-23), is central to development of autoimmune diabetes of the NOD mouse model. We hypothesized that enhanced susceptibility to autoimmune diabetes is the result of targeting of insulin by a T-cell receptor (TCR) sequence commonly encoded in the germline. In this study, we aimed to demonstrate that a particular Vα gene TRAV5D-4 with multiple junction sequences is sufficient to induce anti-islet autoimmunity by studying retrogenic mouse lines expressing α-chains with different Vα TRAV genes. Retrogenic NOD strains expressing Vα TRAV5D-4 α-chains with many different complementarity determining region (CDR) 3 sequences, even those derived from TCRs recognizing islet-irrelevant molecules, developed anti-insulin autoimmunity. Induction of insulin autoantibodies by TRAV5D-4 α-chains was abrogated by the mutation of insulin peptide B:9-23 or that of two amino acid residues in CDR1 and 2 of the TRAV5D-4. TRAV13-1, the human ortholog of murine TRAV5D-4, was also capable of inducing in vivo anti-insulin autoimmunity when combined with different murine CDR3 sequences. Targeting primary autoantigenic peptides by simple germline-encoded TCR motifs may underlie enhanced susceptibility to the development of autoimmune diabetes.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- R00 DK080885/DK/NIDDK NIH HHS/United States
- K99-DK-080885/DK/NIDDK NIH HHS/United States
- P30-DK-057516/DK/NIDDK NIH HHS/United States
- N01 AI015416/AI/NIAID NIH HHS/United States
- K99 DK080885/DK/NIDDK NIH HHS/United States
- R01 DK081166/DK/NIDDK NIH HHS/United States
- R01 DK055969/DK/NIDDK NIH HHS/United States
- N01-AI-015416/AI/NIAID NIH HHS/United States
- R01 GM-083127/GM/NIGMS NIH HHS/United States
- R01 GM083127/GM/NIGMS NIH HHS/United States
- P30 DK057516/DK/NIDDK NIH HHS/United States
- U19-AI-050864/AI/NIAID NIH HHS/United States
- LM009451/LM/NLM NIH HHS/United States
- T15 LM009451/LM/NLM NIH HHS/United States
- R01-DK-055969/DK/NIDDK NIH HHS/United States
- U19 AI050864/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

