Bioactives of Artemisia dracunculus L. mitigate the role of ceramides in attenuating insulin signaling in rat skeletal muscle cells
- PMID: 22315320
- PMCID: PMC3282822
- DOI: 10.2337/db11-0396
Bioactives of Artemisia dracunculus L. mitigate the role of ceramides in attenuating insulin signaling in rat skeletal muscle cells
Abstract
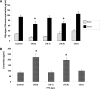
Ectopic lipids in peripheral tissues have been implicated in attenuating insulin action in vivo. The botanical extract of Artemisia dracunculus L. (PMI 5011) improves insulin action, yet the precise mechanism is not known. We sought to determine whether the mechanism by which PMI 5011 improves insulin signaling is through regulation of lipid metabolism. After differentiation, cells were separately preincubated with free fatty acids (FFAs) and ceramide C2, and the effects on glycogen content, insulin signaling, and ceramide profiles were determined. The effect of PMI 5011 on ceramide accumulation and ceramide-induced inhibition of insulin signaling was evaluated. FFAs resulted in increased levels of total ceramides and ceramide species in L6 myotubes. Saturated FFAs and ceramide C2 inhibited insulin-stimulated phosphorylation of protein kinase B/Akt and reduced glycogen content. PMI 5011 had no effect on ceramide formation or accumulation but increased insulin sensitivity via restoration of Akt phosphorylation. PMI 5011 also attenuated the FFA-induced upregulation of a negative inhibitor of insulin signaling, i.e., protein tyrosine phosphatase 1B (PTP1B), and increased phosphorylation of PTP1B. PMI 5011 attenuates the reduction in insulin signaling induced by ceramide accumulation, but the mechanism of improved insulin signaling is independent of ceramide formation.
Figures

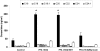


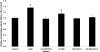


References
-
- Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008;7:45–56 - PubMed
-
- Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys 2003;419:101–109 - PubMed
-
- Chavez JA, Knotts TA, Wang LP, et al. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem 2003;278:10297–10303 - PubMed
-
- Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res 2006;45:42–72 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

