Plasmacytoid dendritic cells control T-cell response to chronic viral infection
- PMID: 22315415
- PMCID: PMC3286988
- DOI: 10.1073/pnas.1117359109
Plasmacytoid dendritic cells control T-cell response to chronic viral infection
Abstract
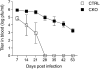
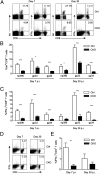
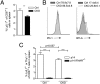
Infections with persistent viruses are a frequent cause of immunosuppression, autoimmune sequelae, and/or neoplastic disease. Plasmacytoid dendritic cells (pDCs) are innate immune cells that produce type I interferon (IFN-I) and other cytokines in response to virus-derived nucleic acids. Persistent viruses often cause depletion or functional impairment of pDCs, but the role of pDCs in the control of these viruses remains unclear. We used conditional targeting of pDC-specific transcription factor E2-2 to generate mice that constitutively lack pDCs in peripheral lymphoid organs and tissues. The profound impact of pDC deficiency on innate antiviral responses was revealed by the failure to control acute infection with the cytopathic mouse hepatitis virus. Furthermore, pDC-deficient animals failed to clear lymphocytic choriomeningitis virus (LCMV) from hematopoietic organs during persistent LCMV infection. This failure was associated with reduced numbers and functionality of LCMV-specific CD4(+) helper T cells and impaired antiviral CD8(+) T-cell responses. Adoptive transfer of LCMV-specific T cells revealed that both CD4(+) and CD8(+) T cells required IFN-I for expansion, but only CD4(+) T cells required the presence of pDCs. In contrast, mice with pDC-specific loss of MHC class II expression supported normal CD4(+) T-cell response to LCMV. These data suggest that pDCs facilitate CD4(+) helper T-cell responses to persistent viruses independently of direct antigen presentation. Thus pDCs provide an essential link between innate and adaptive immunity to chronic viral infection, likely through the secretion of IFN-I and other cytokines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Klenerman P, Hill A. T cells and viral persistence: Lessons from diverse infections. Nat Immunol. 2005;6:873–879. - PubMed
-
- Buchmeier MJ, Welsh RM, Dutko FJ, Oldstone MB. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. - PubMed
-
- Moskophidis D, Lechner F, Hengartner H, Zinkernagel RM. MHC class I and non-MHC-linked capacity for generating an anti-viral CTL response determines susceptibility to CTL exhaustion and establishment of virus persistence in mice. J Immunol. 1994;152:4976–4983. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

