Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane
- PMID: 22315430
- PMCID: PMC3286910
- DOI: 10.1073/pnas.1200718109
Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane
Abstract
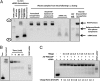
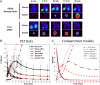
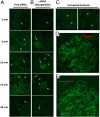
Despite being engineered to avoid renal clearance, many cationic polymer (polycation)-based siRNA nanoparticles that are used for systemic delivery are rapidly eliminated from the circulation. Here, we show that a component of the renal filtration barrier--the glomerular basement membrane (GBM)--can disassemble cationic cyclodextrin-containing polymer (CDP)-based siRNA nanoparticles and, thereby, facilitate their rapid elimination from circulation. Using confocal and electron microscopies, positron emission tomography, and compartment modeling, we demonstrate that siRNA nanoparticles, but not free siRNA, accumulate and disassemble in the GBM. We also confirm that the siRNA nanoparticles do not disassemble in blood plasma in vitro and in vivo. This clearance mechanism may affect any nanoparticles that assemble primarily by electrostatic interactions between cationic delivery components and anionic nucleic acids (or other therapeutic entities).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–782. - PubMed
-
- de Wolf HK, et al. Effect of cationic carriers on the pharmacokinetics and tumor localization of nucleic acids after intravenous administration. Int J Pharm. 2007;331:167–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

