Presenilins and γ-secretase: structure, function, and role in Alzheimer Disease
- PMID: 22315713
- PMCID: PMC3253024
- DOI: 10.1101/cshperspect.a006304
Presenilins and γ-secretase: structure, function, and role in Alzheimer Disease
Abstract
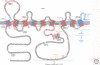
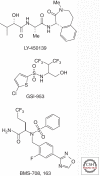
Presenilins were first discovered as sites of missense mutations responsible for early-onset Alzheimer disease (AD). The encoded multipass membrane proteins were subsequently found to be the catalytic components of γ-secretases, membrane-embedded aspartyl protease complexes responsible for generating the carboxyl terminus of the amyloid β-protein (Aβ) from the amyloid protein precursor (APP). The protease complex also cleaves a variety of other type I integral membrane proteins, most notably the Notch receptor, signaling from which is involved in many cell differentiation events. Although γ-secretase is a top target for developing disease-modifying AD therapeutics, interference with Notch signaling should be avoided. Compounds that alter Aβ production by γ-secretase without affecting Notch proteolysis and signaling have been identified and are currently at various stages in the drug development pipeline.
Figures



References
-
- Alzheimer’s Disease Collaborative Group 1995. The structure of the presenilin 1 (S182) gene and identification of six novel mutations in early onset AD families. Nat Genet 11: 219–222 - PubMed
-
- Baumann K, Paganetti PA, Sturchler-Pierrat C, Wong C, Hartmann H, Cescato R, Frey P, Yankner BA, Sommer B, Staufenbiel M 1997. Distinct processing of endogenous and overexpressed recombinant presenilin 1. Neurobiol Aging 18: 181–189 - PubMed
-
- Beher D, Fricker M, Nadin A, Clarke EE, Wrigley JD, Li YM, Culvenor JG, Masters CL, Harrison T, Shearman MS 2003. In vitro characterization of the presenilin-dependent γ-secretase complex using a novel affinity ligand. Biochemistry 427: 8133–8142 - PubMed
-
- Beher D, Clarke EE, Wrigley JD, Martin AC, Nadin A, Churcher I, Shearman MS 2004. Selected non-steroidal anti-inflammatory drugs and their derivatives target γ-secretase at a novel site. Evidence for an allosteric mechanism. J Biol Chem 279: 43419–43426 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
