Dysfunction of the RAR/RXR signaling pathway in the forebrain impairs hippocampal memory and synaptic plasticity
- PMID: 22316320
- PMCID: PMC3298701
- DOI: 10.1186/1756-6606-5-8
Dysfunction of the RAR/RXR signaling pathway in the forebrain impairs hippocampal memory and synaptic plasticity
Abstract
Background: Retinoid signaling pathways mediated by retinoic acid receptor (RAR)/retinoid × receptor (RXR)-mediated transcription play critical roles in hippocampal synaptic plasticity. Furthermore, recent studies have shown that treatment with retinoic acid alleviates age-related deficits in hippocampal long-term potentiation (LTP) and memory performance and, furthermore, memory deficits in a transgenic mouse model of Alzheimer's disease. However, the roles of the RAR/RXR signaling pathway in learning and memory at the behavioral level have still not been well characterized in the adult brain. We here show essential roles for RAR/RXR in hippocampus-dependent learning and memory. In the current study, we generated transgenic mice in which the expression of dominant-negative RAR (dnRAR) could be induced in the mature brain using a tetracycline-dependent transcription factor and examined the effects of RAR/RXR loss.
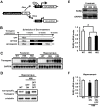
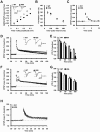
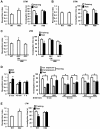
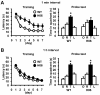
Results: The expression of dnRAR in the forebrain down-regulated the expression of RARβ, a target gene of RAR/RXR, indicating that dnRAR mice exhibit dysfunction of the RAR/RXR signaling pathway. Similar with previous findings, dnRAR mice displayed impaired LTP and AMPA-mediated synaptic transmission in the hippocampus. More importantly, these mutant mice displayed impaired hippocampus-dependent social recognition and spatial memory. However, these deficits of LTP and memory performance were rescued by stronger conditioning stimulation and spaced training, respectively. Finally, we found that pharmacological blockade of RARα in the hippocampus impairs social recognition memory.
Conclusions: From these observations, we concluded that the RAR/RXR signaling pathway greatly contributes to learning and memory, and LTP in the hippocampus in the adult brain.
Figures
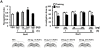
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

