Evaluation of blood-brain barrier transport and CNS drug metabolism in diseased and control brain after intravenous L-DOPA in a unilateral rat model of Parkinson's disease
- PMID: 22316420
- PMCID: PMC3298802
- DOI: 10.1186/2045-8118-9-4
Evaluation of blood-brain barrier transport and CNS drug metabolism in diseased and control brain after intravenous L-DOPA in a unilateral rat model of Parkinson's disease
Abstract
Background: Changes in blood-brain barrier (BBB) functionality have been implicated in Parkinson's disease. This study aimed to investigate BBB transport of L-DOPA transport in conjunction with its intra-brain conversion, in both control and diseased cerebral hemispheres in the unilateral rat rotenone model of Parkinson's disease.
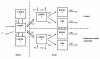
Methods: In Lewis rats, at 14 days after unilateral infusion of rotenone into the medial forebrain bundle, L-DOPA was administered intravenously (10, 25 or 50 mg/kg). Serial blood samples and brain striatal microdialysates were analysed for L-DOPA, and the dopamine metabolites DOPAC and HVA. Ex-vivo brain tissue was analyzed for changes in tyrosine hydroxylase staining as a biomarker for Parkinson's disease severity. Data were analysed by population pharmacokinetic analysis (NONMEM) to compare BBB transport of L-DOPA in conjunction with the conversion of L-DOPA into DOPAC and HVA, in control and diseased cerebral hemisphere.
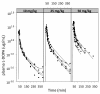
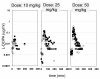
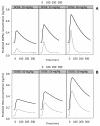
Results: Plasma pharmacokinetics of L-DOPA could be described by a 3-compartmental model. In rotenone responders (71%), no difference in L-DOPA BBB transport was found between diseased and control cerebral hemisphere. However, in the diseased compared with the control side, basal microdialysate levels of DOPAC and HVA were substantially lower, whereas following L-DOPA administration their elimination rates were higher.
Conclusions: Parkinson's disease-like pathology, indicated by a huge reduction of tyrosine hydroxylase as well as by substantially reduced levels and higher elimination rates of DOPAC and HVA, does not result in changes in BBB transport of L-DOPA. Taking the results of this study and that of previous ones, it can be concluded that changes in BBB functionality are not a specific characteristic of Parkinson's disease, and cannot account for the decreased benefit of L-DOPA at later stages of Parkinson's disease.
Figures

References
-
- Männistö PT, Ulmanen I, Lundström K, Taskinen J, Tenhunen J, Tilgmann C, Kaakkola S. Characteristics of catechol O-methyl-transferase (COMT) and properties of selective COMT inhibitors. Prog Drug Res. 1992;39:291–350. - PubMed
LinkOut - more resources
Full Text Sources

