Plasmacytoid dendritic cells reduce HIV production in elite controllers
- PMID: 22318133
- PMCID: PMC3318617
- DOI: 10.1128/JVI.07114-11
Plasmacytoid dendritic cells reduce HIV production in elite controllers
Abstract
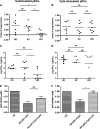
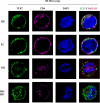
HIV elite controllers (EC) are a rare group of HIV-infected patients who are able to maintain undetectable viral loads during a long period of time in the absence of antiretroviral treatment. Adaptive immunity and host genetic factors, although implicated, do not entirely explain this phenomenon. On the other hand, plasmacytoid dendritic cells (pDCs) are the principal type I interferon (IFN) producers in response to viral infection, and it is unknown whether pDCs are involved in the control of HIV infection in EC. In our study, we analyzed peripheral pDC levels and IFN-α production by peripheral blood mononuclear cells (PBMCs) in EC compared to other groups of HIV-infected patients, the ability of pDCs to reduce HIV production in vitro, and the mechanisms potentially involved. We showed preserved pDC counts and IFN-α production in EC. We also observed a higher capacity of pDCs from EC to reduce HIV production and to induce T cell apoptosis, whereas pDCs from viremic patients barely responded without previous Toll-like receptor 9 (TLR-9) stimulus. The preserved functionality of pDCs from EC to reduce viral production may be one of the mechanisms involved in the control of HIV viremia in these subjects. These results demonstrate the importance of innate immunity in HIV pathogenesis, and an understanding of pDC mechanisms would be helpful for the design of new therapies.
Figures


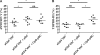
References
-
- Deeks SG, Walker BD. 2007. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 27:406–416 - PubMed
-
- Finke JS, Shodell M, Shah K, Siegal FP, Steinman RM. 2004. Dendritic cell numbers in the blood of HIV-1 infected patients before and after changes in antiretroviral therapy. J. Clin. Immunol. 24:647–652 - PubMed
-
- Gurney KB, Colantonio AD, Blom B, Spits H, Uittenbogaart CH. 2004. Endogenous IFN-alpha production by plasmacytoid dendritic cells exerts an antiviral effect on thymic HIV-1 infection. J. Immunol. 173:7269–7276 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

