Enhanced susceptibility of Ago1/3 double-null mice to influenza A virus infection
- PMID: 22318144
- PMCID: PMC3318639
- DOI: 10.1128/JVI.05303-11
Enhanced susceptibility of Ago1/3 double-null mice to influenza A virus infection
Abstract
RNA interference (RNAi) is a critical component of many cellular antiviral responses in plants, invertebrates, and mammals. However, its in vivo role in host protection from the negative-sense RNA virus influenza virus type A (flu) is unclear. Here we have examined the role of RNAi in host defense to flu by analyzing Argonaute 1 and 3 double-knockout mice deficient in components of the RNA-induced silencing complex. Compared to littermate controls, flu-infected double-knockout mice exhibited increased mortality, consistent with more severe alveolitis and pneumonitis. These data indicate that optimal resistance to flu requires Argonaute 1 and/or 3. Enhanced mortality of double-knockout mice was not associated either with increased viral replication or with differential pulmonary recruitment or function of innate and adaptive immune cells. Given the absence of detectable immune defects, our results support the notion that the enhanced flu susceptibility of double-knockout mice arises from an intrinsic impairment in the ability of lung cells to tolerate flu-elicited inflammation.
Figures
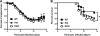
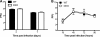
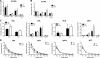

References
-
- Beitzinger M, Peters L, Zhu JY, Kremmer E, Meister G. 2007. Identification of human microRNA targets from isolated argonaute protein complexes. RNA Biol. 4:76–84 - PubMed
-
- Bernstein E, et al. 2003. Dicer is essential for mouse development. Nat. Genet. 35:215–217 - PubMed
-
- Calin GA, Croce CM. 2006. MicroRNA signatures in human cancers. Nat. Rev. Cancer 6:857–866 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

