N-acetylcysteine reduces oxidative stress in sickle cell patients
- PMID: 22318468
- PMCID: PMC3368118
- DOI: 10.1007/s00277-011-1404-z
N-acetylcysteine reduces oxidative stress in sickle cell patients
Abstract
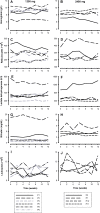
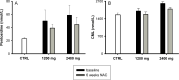
Oxidative stress is of importance in the pathophysiology of sickle cell disease (SCD). In this open label randomized pilot study the effects of oral N-acetylcysteine (NAC) on phosphatidylserine (PS) expression as marker of cellular oxidative damage (primary end point), and markers of hemolysis, coagulation and endothelial activation and NAC tolerability (secondary end points) were studied. Eleven consecutive patients (ten homozygous [HbSS] sickle cell patients, one HbSβ(0)-thalassemia patient) were randomly assigned to treatment with either 1,200 or 2,400 mg NAC daily during 6 weeks. The data indicate an increment in whole blood glutathione levels and a decrease in erythrocyte outer membrane phosphatidylserine exposure, plasma levels of advanced glycation end-products (AGEs) and cell-free hemoglobin after 6 weeks of NAC treatment in both dose groups. One patient did not tolerate the 2,400 mg dose and continued with the 1,200 mg dose. During the study period, none of the patients experienced painful crises or other significant SCD or NAC related complications. These data indicate that N-acetylcysteine treatment of sickle cell patients may reduce SCD related oxidative stress.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

