Oral tolerance to food protein
- PMID: 22318493
- PMCID: PMC3328017
- DOI: 10.1038/mi.2012.4
Oral tolerance to food protein
Abstract
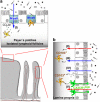
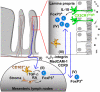
Oral tolerance is the state of local and systemic immune unresponsiveness that is induced by oral administration of innocuous antigen such as food proteins. An analogous but more local process also regulates responses to commensal bacteria in the large intestine and, together, mucosally induced tolerance appears to prevent intestinal disorders such as food allergy, celiac disease, and inflammatory bowel diseases. Here we discuss the anatomical basis of antigen uptake and recognition in oral tolerance and highlight possible mechanisms underlying the immunosuppression. We propose a model of stepwise induction of oral tolerance in which specialized populations of mucosal dendritic cells and the unique microenvironment of draining mesenteric lymph nodes combine to generate regulatory T cells that undergo subsequent expansion in the small intestinal lamina propria. The local and systemic effects of these regulatory T cells prevent potentially dangerous hypersensitivity reactions to harmless antigens derived from the intestine and hence are crucial players in immune homeostasis.
Figures

References
-
- Molodecky N.A., et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2011;142,:46–54.e42. - PubMed
-
- Meresse B., Ripoche J., Heyman M., Cerf-Bensussan N. Celiac disease: from oral tolerance to intestinal inflammation, autoimmunity and lymphomagenesis. Mucosal Immunol. 2009;2,:8–23. - PubMed
-
- Wells H.G., Osborne T.B. The biological reactions of the vegetable proteins. I: Anaphylaxis. J. Infect. Dis. 1911;8,:66–124.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

