Expression of tumour-specific antigens underlies cancer immunoediting
- PMID: 22318517
- PMCID: PMC3288744
- DOI: 10.1038/nature10803
Expression of tumour-specific antigens underlies cancer immunoediting
Abstract
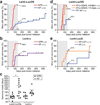
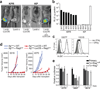
Cancer immunoediting is a process by which immune cells, particularly lymphocytes of the adaptive immune system, protect the host from the development of cancer and alter tumour progression by driving the outgrowth of tumour cells with decreased sensitivity to immune attack. Carcinogen-induced mouse models of cancer have shown that primary tumour susceptibility is thereby enhanced in immune-compromised mice, whereas the capacity for such tumours to grow after transplantation into wild-type mice is reduced. However, many questions about the process of cancer immunoediting remain unanswered, in part because of the known antigenic complexity and heterogeneity of carcinogen-induced tumours. Here we adapted a genetically engineered, autochthonous mouse model of sarcomagenesis to investigate the process of cancer immunoediting. This system allows us to monitor the onset and growth of immunogenic and non-immunogenic tumours induced in situ that harbour identical genetic and histopathological characteristics. By comparing the development of such tumours in immune-competent mice with their development in mice with broad immunodeficiency or specific antigenic tolerance, we show that recognition of tumour-specific antigens by lymphocytes is critical for immunoediting against sarcomas. Furthermore, primary sarcomas were edited to become less immunogenic through the selective outgrowth of cells that were able to escape T lymphocyte attack. Loss of tumour antigen expression or presentation on major histocompatibility complex I was necessary and sufficient for this immunoediting process to occur. These results highlight the importance of tumour-specific-antigen expression in immune surveillance, and potentially, immunotherapy.
Figures


Comment in
-
Tumour immunogenicity: editorial selection demystified.Nat Rev Cancer. 2012 Mar 22;12(4):227. doi: 10.1038/nrc3251. Nat Rev Cancer. 2012. PMID: 22437866 No abstract available.
-
Tumour immunology: Editorial selection demystified.Nat Rev Immunol. 2012 Mar 22;12(4):233. doi: 10.1038/nri3202. Nat Rev Immunol. 2012. PMID: 22437936 No abstract available.
References
-
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. - PubMed
-
- Shankaran V, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. - PubMed
-
- Qin Z, Blankenstein T. A cancer immunosurveillance controversy. Nat Immunol. 2004;5:3–4. author reply 4–5. - PubMed
-
- Kirsch DG, et al. A spatially and temporally restricted mouse model of soft tissue sarcoma. Nat Med. 2007;13:992–997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

