Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting
- PMID: 22318521
- PMCID: PMC3874809
- DOI: 10.1038/nature10755
Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting
Abstract
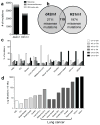
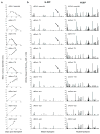
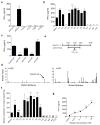
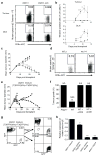
Cancer immunoediting, the process by which the immune system controls tumour outgrowth and shapes tumour immunogenicity, is comprised of three phases: elimination, equilibrium and escape. Although many immune components that participate in this process are known, its underlying mechanisms remain poorly defined. A central tenet of cancer immunoediting is that T-cell recognition of tumour antigens drives the immunological destruction or sculpting of a developing cancer. However, our current understanding of tumour antigens comes largely from analyses of cancers that develop in immunocompetent hosts and thus may have already been edited. Little is known about the antigens expressed in nascent tumour cells, whether they are sufficient to induce protective antitumour immune responses or whether their expression is modulated by the immune system. Here, using massively parallel sequencing, we characterize expressed mutations in highly immunogenic methylcholanthrene-induced sarcomas derived from immunodeficient Rag2(-/-) mice that phenotypically resemble nascent primary tumour cells. Using class I prediction algorithms, we identify mutant spectrin-β2 as a potential rejection antigen of the d42m1 sarcoma and validate this prediction by conventional antigen expression cloning and detection. We also demonstrate that cancer immunoediting of d42m1 occurs via a T-cell-dependent immunoselection process that promotes outgrowth of pre-existing tumour cell clones lacking highly antigenic mutant spectrin-β2 and other potential strong antigens. These results demonstrate that the strong immunogenicity of an unedited tumour can be ascribed to expression of highly antigenic mutant proteins and show that outgrowth of tumour cells that lack these strong antigens via a T-cell-dependent immunoselection process represents one mechanism of cancer immunoediting.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Comment in
-
Tumour immunogenicity: editorial selection demystified.Nat Rev Cancer. 2012 Mar 22;12(4):227. doi: 10.1038/nrc3251. Nat Rev Cancer. 2012. PMID: 22437866 No abstract available.
-
Tumour immunology: Editorial selection demystified.Nat Rev Immunol. 2012 Mar 22;12(4):233. doi: 10.1038/nri3202. Nat Rev Immunol. 2012. PMID: 22437936 No abstract available.
-
T-cell dependent immunoselection.Oncoimmunology. 2012 Oct 1;1(7):1003. doi: 10.4161/onci.20927. Oncoimmunology. 2012. PMID: 23170248 Free PMC article. No abstract available.
References
-
- Shankaran V, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–11. - PubMed
-
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. - PubMed
-
- Koebel CM, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–7. - PubMed
-
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural Innate and Adaptive Immunity to Cancer. Annu Rev Immunol. 2011;29:235–271. - PubMed
-
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

