Initiation of synapse formation by Wnt-induced MuSK endocytosis
- PMID: 22318632
- PMCID: PMC3274363
- DOI: 10.1242/dev.071555
Initiation of synapse formation by Wnt-induced MuSK endocytosis
Abstract
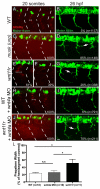
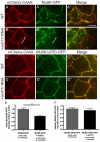
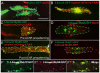
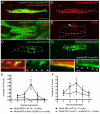
In zebrafish, the MuSK receptor initiates neuromuscular synapse formation by restricting presynaptic growth cones and postsynaptic acetylcholine receptors (AChRs) to the center of skeletal muscle cells. Increasing evidence suggests a role for Wnts in this process, yet how muscle cells respond to Wnt signals is unclear. Here, we show that in vivo, wnt11r and wnt4a initiate MuSK translocation from muscle membranes to recycling endosomes and that this transition is crucial for AChR accumulation at future synaptic sites. Moreover, we demonstrate that components of the planar cell polarity pathway colocalize to recycling endosomes and that this localization is MuSK dependent. Knockdown of several core components disrupts MuSK translocation to endosomes, AChR localization and axonal guidance. We propose that Wnt-induced trafficking of the MuSK receptor to endosomes initiates a signaling cascade to align pre- with postsynaptic elements. Collectively, these findings suggest a general mechanism by which Wnt signals shape synaptic connectivity through localized receptor endocytosis.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

