Rpn1 and Rpn2 coordinate ubiquitin processing factors at proteasome
- PMID: 22318722
- PMCID: PMC3340268
- DOI: 10.1074/jbc.M111.316323
Rpn1 and Rpn2 coordinate ubiquitin processing factors at proteasome
Abstract
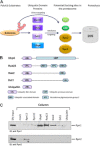
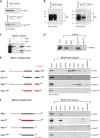
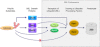
Substrates tagged with (poly)ubiquitin for degradation can be targeted directly to the 26 S proteasome where they are proteolyzed. Independently, ubiquitin conjugates may also be delivered by bivalent shuttles. The majority of shuttles attach to the proteasome through a ubiquitin-like domain (UBL) while anchoring cargo at a C-terminal polyubiquitin-binding domain(s). We found that two shuttles of this class, Rad23 and Dsk2, dock at two different receptor sites embedded within a single subunit of the 19 S proteasome regulatory particle, Rpn1. Their association/dissociation constants and affinities for Rpn1 are similar. In contrast, another UBL-containing protein, the deubiquitinase Ubp6, is also anchored by Rpn1, yet it dissociates slower, thus behaving as an occasional proteasome subunit that is distinct from the transiently associated shuttles. Two neighboring subunits, Rpn10 and Rpn13, show a marked preference for polyubiquitin over UBLs. Rpn10 attaches to the central solenoid portion of Rpn1, although this association is stabilized by the presence of a third subunit, Rpn2. Rpn13 binds directly to Rpn2. These intrinsic polyubiquitin receptors may compete with substrate shuttles for their polyubiquitin-conjugate cargos, thereby aiding release of the emptied shuttles. By binding multiple ubiquitin-processing factors simultaneously, Rpn1 is uniquely suited to coordinate substrate recruitment, deubiquitination, and movement toward the catalytic core. The broad range of affinities for ubiquitin, ubiquitin-like, and non-ubiquitin signals by adjacent yet nonoverlapping sites all within the base represents a hub of activity that coordinates the intricate relay of substrates within the proteasome, and consequently it influences substrate residency time and commitment to degradation.
Figures
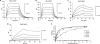


References
-
- Glickman M. H., Rubin D. M., Coux O., Wefes I., Pfeifer G., Cjeka Z., Baumeister W., Fried V. A., Finley D. (1998) A subcomplex of the proteasome regulatory particle required for ubiquitin-conjugate degradation and related to the COP9-signalosome and eIF3. Cell 94, 615–623 - PubMed
-
- Guterman A., Glickman M. H. (2004) Complementary roles for Rpn11 and Ubp6 in deubiquitination and proteolysis by the proteasome. J. Biol. Chem. 279, 1729–1738 - PubMed
-
- Bar-Nun S., Glickman M. H. (2011) Proteosomal AAA-ATPases. Structure and function. Biochim. Biophys. Acta 1823, 67–82 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

