Complement activation in acetaminophen-induced liver injury in mice
- PMID: 22319198
- PMCID: PMC3336815
- DOI: 10.1124/jpet.111.189837
Complement activation in acetaminophen-induced liver injury in mice
Abstract
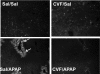
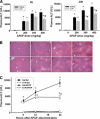
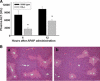
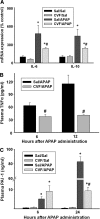
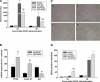
Overdose with acetaminophen (APAP) results in acute liver failure in humans and experimental animals. Complement comprises more than 30 proteins that can participate in tissue injury and/or repair, but the role of complement activation in APAP-induced hepatotoxicity has not been evaluated. Treatment of male, C57BL6J mice with APAP (200-400 mg/kg) resulted in liver injury as evidenced by increased activity of alanine aminotransferase (ALT) in plasma and hepatocellular necrosis. Plasma concentration of the complement component C3 was significantly reduced 6 h after treatment with APAP, indicating complement activation, and C3b (detected by immunostaining) accumulated in the centrilobular areas of liver lobules. Pretreatment with cobra venom factor (CVF; 15 U/mouse) to deplete complement components abolished APAP-mediated C3b accumulation, and this was accompanied by reductions in plasma ALT activity, hepatocellular necrosis, hepatic neutrophil accumulation, and expression of inflammatory genes (interleukin-6, interleukin-10, and plasminogen activation inhibitor-1) at 24 h after APAP treatment. Loss of hepatocellular GSH was similar in APAP-treated mice pretreated with either saline or CVF, suggesting that CVF pretreatment did not affect APAP bioactivation. Mice with a genetic deficiency in C3 had reduced ALT activity 6 and 12 h after APAP administration compared with wild-type animals. These results reveal a key role for complement activation in hepatic inflammation and progression of injury during the pathogenesis of APAP-induced hepatotoxicity.
Figures
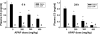
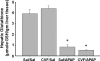

References
-
- Alper CA, Balavitch D. (1976) Cobra venom factor: evidence for its being altered cobra C3 (the third component of complement). Science 191:1275–1276 - PubMed
-
- Bianchi ME. (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81:1–5 - PubMed
-
- Blazka ME, Wilmer JL, Holladay SD, Wilson RE, Luster MI. (1995) Role of proinflammatory cytokines in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 133:43–52 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

